Exam 10: Acids and Bases in Our Environment
Exam 1: About Science65 Questions
Exam 2: Particles of Matter104 Questions
Exam 3: Elements of Chemistry116 Questions
Exam 4: Subatomic Particles146 Questions
Exam 5: The Atomic Nucleus128 Questions
Exam 6: How Atoms Bond153 Questions
Exam 7: How Molecules Mix157 Questions
Exam 8: How Water Behaves140 Questions
Exam 9: How Chemicals React150 Questions
Exam 10: Acids and Bases in Our Environment135 Questions
Exam 11: Oxidations and Reductions Charge the World138 Questions
Exam 12: Organic Compounds162 Questions
Exam 13: Nutrients of Life138 Questions
Exam 14: Medicinal Chemistry85 Questions
Exam 15: Optimizing Food Production115 Questions
Exam 16: Protection Water and Air Resources147 Questions
Exam 17: Capturing Energy93 Questions
Select questions type
What is the relationship between the hydroxide ion and a water molecule?
(Multiple Choice)
4.9/5  (44)
(44)
What happens to the corrosive properties of an acid and a base after they neutralize each other? Why?
(Multiple Choice)
4.9/5  (40)
(40)
If you had a 1 M solution of a strong base what would be its pH?
(Multiple Choice)
4.8/5  (31)
(31)
When a hydronium ion concentration equals 1 × 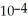 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
moles per liter, what is the pH of the solution? Is the solution acidic or basic?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following statements about strong or weak bases is true?
(Multiple Choice)
5.0/5  (41)
(41)
Which of the following statements about strong and weak acids is not true?
(Multiple Choice)
4.8/5  (41)
(41)
Why is carbon dioxide able to be stored more effectively in ocean water vs. fresh water?
(Multiple Choice)
4.8/5  (43)
(43)
Which of the above illustrations shows a basic aqueous solution?
(Multiple Choice)
4.8/5  (35)
(35)
If you had a 1 M solution of a weak acid what would be its pH?
(Multiple Choice)
4.9/5  (36)
(36)
Identify the acid or base behavior of each substance in these reactions: HS 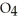
 +
+ 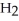 O ⇌ O
O ⇌ O 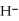 +
+ 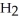 S
S 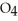
(Multiple Choice)
4.9/5  (41)
(41)
Lakes lying in granite basins, such as those in the northeastern U.S., tend to become acidified by acid rain more readily than lakes lying in limestone basins, such as those found in the midwestern U.S. Why is this so?
(Multiple Choice)
4.7/5  (41)
(41)
As the hydronium ion concentration increases, the pH ________.
(Multiple Choice)
4.8/5  (30)
(30)
When the hydronium ion concentration equals 1 mole per liter, what is the pH of the solution? Is the solution acidic or basic?
(Multiple Choice)
4.9/5  (39)
(39)
What atom in the hydronium ion, H3O⁺, bears the positive charge?
(Multiple Choice)
4.9/5  (42)
(42)
Qualitatively, what happens to the hydroxide ion concentration if you decrease the hydronium ion concentration?
(Multiple Choice)
4.7/5  (43)
(43)
Showing 81 - 100 of 135
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)