Exam 29: Particles and Waves
Exam 1: Introduction and Mathematical Concepts70 Questions
Exam 2: Kinematics in One Dimension103 Questions
Exam 3: Kinematics in Two Dimensions68 Questions
Exam 4: Forces and Newtons Laws of Motion103 Questions
Exam 5: Dynamics of Uniform Circular Motion59 Questions
Exam 6: Work and Energy78 Questions
Exam 7: Impulse and Momentum66 Questions
Exam 8: Rotational Kinematics55 Questions
Exam 9: Rotational Dynamics57 Questions
Exam 10: Simple Harmonic Motion and Elasticity63 Questions
Exam 11: Fluids65 Questions
Exam 12: Temperature and Heat66 Questions
Exam 13: The Transfer of Heat42 Questions
Exam 14: The Ideal Gas Law and Kinetic Theory55 Questions
Exam 15: Thermodynamics79 Questions
Exam 16: Waves and Sound67 Questions
Exam 17: The Principle of Linear Superposition and Interference Phenomena46 Questions
Exam 18: Electric Forces and Electric Fields61 Questions
Exam 19: Electric Potential Energy and the Electric Potential70 Questions
Exam 20: Electric Circuits100 Questions
Exam 21: Magnetic Forces and Magnetic Fields66 Questions
Exam 22: Electromagnetic Induction71 Questions
Exam 23: Alternating Current Circuits84 Questions
Exam 24: Electromagnetic Waves66 Questions
Exam 25: The Refl Ection of Light: Mirrors43 Questions
Exam 26: The Refraction of Light: Lenses and Optical Instruments102 Questions
Exam 27: Interference and the Wave Nature of Light57 Questions
Exam 28: Special Relativity63 Questions
Exam 29: Particles and Waves54 Questions
Exam 30: The Nature of the Atom74 Questions
Exam 31: Nuclear Physics and Radioactivity37 Questions
Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles45 Questions
Select questions type
A photon of wavelength 200 nm is scattered by an electron that is initially at rest. Which one of the following statements concerning the wavelength of the scattered photon is true?
(Multiple Choice)
4.7/5  (41)
(41)
It is desired to obtain a diffraction pattern for electrons using a diffraction grating with lines separated by 10 nm. The mass of an electron is 9.11 × 10-31 kg.
-Suppose it is desired to observe diffraction effects for a beam of electromagnetic radiation using the same grating. Roughly, what is the required energy of the individual photons in the beam?
(Multiple Choice)
4.9/5  (41)
(41)
The speed of a bullet with a mass of 0.050 kg is 420 m/s with an uncertainty of 0.010 %. What is the minimum uncertainty in the bullet's position if it is measured at the same time as the speed is measured?
(Multiple Choice)
4.7/5  (38)
(38)
For which one of the following problems did Max Planck make contributions that eventually led to the development of the "quantum" hypothesis?
(Multiple Choice)
4.9/5  (38)
(38)
Which experimental evidence confirms the hypothesis that matter exhibits wave properties?
(Multiple Choice)
4.8/5  (39)
(39)
Which one of the following phrases best describes the term work function?
(Multiple Choice)
4.8/5  (46)
(46)
The work function for a particular metal is 4.0 eV. Which one of the following best describes the wavelength of electromagnetic radiation needed to eject electrons from this metal?
(Multiple Choice)
4.8/5  (41)
(41)
The de Broglie wavelength of an electron (m = 9.11 × 10-31 kg) is 1.2 × 10-10 m. Determine the kinetic energy of the electron.
(Multiple Choice)
4.9/5  (41)
(41)
In an experiment to determine the speed and position of an electron (m = 9.11 × 10-31 kg), three researchers claim to have measured the position of the electron to within ± 10-9 m. They reported the following values for the speed of the electron: 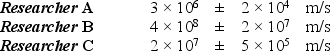 Which of these measurements violates one or more basic laws of modern physics?
Which of these measurements violates one or more basic laws of modern physics?
(Multiple Choice)
4.8/5  (33)
(33)
Determine the energy of a single photon in a monochromatic beam of light of wavelength 625 nm.
(Multiple Choice)
4.9/5  (29)
(29)
A photon has a collision with a stationary electron (h/mc = 2.43 × 10-12 m) and loses 5.0% of its energy. The photon scattering angle is 180°. What is the wavelength of the incident photon in this scattering process?
(Multiple Choice)
4.8/5  (29)
(29)
What is the de Broglie wavelength of an electron (m = 9.11 × 10-31 kg) in a 2.5 × 103-volt X-ray tube?
(Multiple Choice)
4.7/5  (32)
(32)
Approximately, what is the de Broglie wavelength of an electron that has been accelerated through a potential difference of 225 V? The mass of an electron is 9.11 × 10-31 kg.
(Multiple Choice)
4.9/5  (44)
(44)
The Hubble Space Telescope has an orbital speed of 7.56 × 103 m/s and a mass of 11 600 kg. What is the de Broglie wavelength of the telescope?
(Multiple Choice)
4.8/5  (45)
(45)
Showing 41 - 54 of 54
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)