Exam 29: Particles and Waves
Exam 1: Introduction and Mathematical Concepts70 Questions
Exam 2: Kinematics in One Dimension103 Questions
Exam 3: Kinematics in Two Dimensions68 Questions
Exam 4: Forces and Newtons Laws of Motion103 Questions
Exam 5: Dynamics of Uniform Circular Motion59 Questions
Exam 6: Work and Energy78 Questions
Exam 7: Impulse and Momentum66 Questions
Exam 8: Rotational Kinematics55 Questions
Exam 9: Rotational Dynamics57 Questions
Exam 10: Simple Harmonic Motion and Elasticity63 Questions
Exam 11: Fluids65 Questions
Exam 12: Temperature and Heat66 Questions
Exam 13: The Transfer of Heat42 Questions
Exam 14: The Ideal Gas Law and Kinetic Theory55 Questions
Exam 15: Thermodynamics79 Questions
Exam 16: Waves and Sound67 Questions
Exam 17: The Principle of Linear Superposition and Interference Phenomena46 Questions
Exam 18: Electric Forces and Electric Fields61 Questions
Exam 19: Electric Potential Energy and the Electric Potential70 Questions
Exam 20: Electric Circuits100 Questions
Exam 21: Magnetic Forces and Magnetic Fields66 Questions
Exam 22: Electromagnetic Induction71 Questions
Exam 23: Alternating Current Circuits84 Questions
Exam 24: Electromagnetic Waves66 Questions
Exam 25: The Refl Ection of Light: Mirrors43 Questions
Exam 26: The Refraction of Light: Lenses and Optical Instruments102 Questions
Exam 27: Interference and the Wave Nature of Light57 Questions
Exam 28: Special Relativity63 Questions
Exam 29: Particles and Waves54 Questions
Exam 30: The Nature of the Atom74 Questions
Exam 31: Nuclear Physics and Radioactivity37 Questions
Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles45 Questions
Select questions type
A physicist wishes to produce electrons by shining light on a metal surface. The light source emits light with a wavelength of 450 nm. The table lists the only available metals and their work functions. 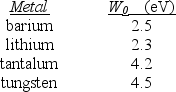 -Which entry in the table below correctly identifies the metal that will produce the most energetic electrons and their energies?
-Which entry in the table below correctly identifies the metal that will produce the most energetic electrons and their energies? 
(Multiple Choice)
4.7/5  (45)
(45)
A physicist wishes to produce electrons by shining light on a metal surface. The light source emits light with a wavelength of 450 nm. The table lists the only available metals and their work functions.  -Which metal(s) can be used to produce electrons by the photoelectric effect?
-Which metal(s) can be used to produce electrons by the photoelectric effect?
(Multiple Choice)
4.8/5  (40)
(40)
What is the speed of an electron that has the same momentum as a photon with a wavelength in vacuum of 488 nm? The mass of an electron is 9.11 × 10-31 kg.
(Multiple Choice)
4.7/5  (36)
(36)
Electrons are emitted from a certain metal with a maximum kinetic energy of 2 eV when 6-eV photons are incident on its surface. If photons of twice the wavelength are incident on this metal which one of the following statements is true?
(Multiple Choice)
5.0/5  (36)
(36)
If Planck's constant were changed to 660 J . s, what would be the minimum uncertainty in the position of a 120-kg football player running at a speed of 3.5 m/s?
(Multiple Choice)
4.8/5  (35)
(35)
In a computer monitor, electrons approach the screen at 1.20 × 108 m/s. What is the de Broglie wavelength of these electrons? Note: the mass of electrons is 9.109 × 10-31 kg; and use the relativistic momentum in your calculation.
(Multiple Choice)
4.8/5  (40)
(40)
Determine the de Broglie wavelength of a neutron (m = 1.67 × 10-27 kg) that has a speed of 5.0 m/s.
(Multiple Choice)
4.9/5  (34)
(34)
In the Compton effect, a photon of wavelength and frequency f hits an electron that is initially at rest. Which one of the following occurs as a result of the collision?
(Multiple Choice)
4.9/5  (42)
(42)
Which one of the following is demonstrated by the Compton effect?
(Multiple Choice)
5.0/5  (35)
(35)
What is the kinetic energy of each electron in a beam of electrons if the beam produces a diffraction pattern of a crystal which is similar to that of a beam of 1.00 eV neutrons? Note: The electron mass is 9.11 × 10-31 kg; and the neutron mass is 1.67 × 10-27 kg.
(Multiple Choice)
4.9/5  (38)
(38)
Complete the following statement: The photon description of light is necessary to explain
(Multiple Choice)
4.8/5  (37)
(37)
What kinetic energy must each neutron in a beam of neutrons have if each has a wavelength of 0.10 nm? The mass of a neutron is 1.67 × 10-27 kg.
(Multiple Choice)
4.7/5  (36)
(36)
An X-ray generator produces photons with energy 49 600 eV or less. Which one of the following phrases most accurately describes the wavelength of these photons?
(Multiple Choice)
4.7/5  (30)
(30)
Incident X-rays in a Compton scattering experiment have a wavelength of 0.400 nm. Determine the wavelength of the scattered X-rays that are detected at an angle of 80.0°. The Compton wavelength of the electron is 2.43 × 10-12 m.
(Multiple Choice)
4.7/5  (34)
(34)
White light consisting of wavelengths 380 nm 750 nm is incident on a lead surface. For which one of the following ranges of wavelengths will photoelectrons be emitted from the lead surface that has a work function W0 = 6.63 × 10-19 J?
(Multiple Choice)
4.8/5  (39)
(39)
A laser emits a single, 3.0-ms pulse of light that has a frequency of 2.83 × 1011 Hz and a total power of 65 000 W. How many photons are in the pulse?
(Multiple Choice)
4.8/5  (39)
(39)
The graph shows the variation in radiation intensity per unit wavelength versus wavelength for a perfect blackbody at temperature T. 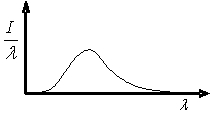 Complete the following statement: As the blackbody temperature is increased, the peak in intensity of this curve
Complete the following statement: As the blackbody temperature is increased, the peak in intensity of this curve
(Multiple Choice)
4.8/5  (42)
(42)
Light is usually thought of as wave-like in nature and electrons as particle-like. In which one of the following instances does light behave as a particle or does an electron behave as a wave?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 21 - 40 of 54
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)