Exam 12: Temperature and Heat
Exam 1: Introduction and Mathematical Concepts70 Questions
Exam 2: Kinematics in One Dimension103 Questions
Exam 3: Kinematics in Two Dimensions68 Questions
Exam 4: Forces and Newtons Laws of Motion103 Questions
Exam 5: Dynamics of Uniform Circular Motion59 Questions
Exam 6: Work and Energy78 Questions
Exam 7: Impulse and Momentum66 Questions
Exam 8: Rotational Kinematics55 Questions
Exam 9: Rotational Dynamics57 Questions
Exam 10: Simple Harmonic Motion and Elasticity63 Questions
Exam 11: Fluids65 Questions
Exam 12: Temperature and Heat66 Questions
Exam 13: The Transfer of Heat42 Questions
Exam 14: The Ideal Gas Law and Kinetic Theory55 Questions
Exam 15: Thermodynamics79 Questions
Exam 16: Waves and Sound67 Questions
Exam 17: The Principle of Linear Superposition and Interference Phenomena46 Questions
Exam 18: Electric Forces and Electric Fields61 Questions
Exam 19: Electric Potential Energy and the Electric Potential70 Questions
Exam 20: Electric Circuits100 Questions
Exam 21: Magnetic Forces and Magnetic Fields66 Questions
Exam 22: Electromagnetic Induction71 Questions
Exam 23: Alternating Current Circuits84 Questions
Exam 24: Electromagnetic Waves66 Questions
Exam 25: The Refl Ection of Light: Mirrors43 Questions
Exam 26: The Refraction of Light: Lenses and Optical Instruments102 Questions
Exam 27: Interference and the Wave Nature of Light57 Questions
Exam 28: Special Relativity63 Questions
Exam 29: Particles and Waves54 Questions
Exam 30: The Nature of the Atom74 Questions
Exam 31: Nuclear Physics and Radioactivity37 Questions
Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles45 Questions
Select questions type
Two spheres, labeled A and B, have identical masses, but are made of different substances. The specific heat capacity of sphere A is 645 J/(kg · C°) and that of sphere B is 240 J/(kg · C°). The spheres are initially at 21 °C; and the same quantity of heat is added to each sphere. If the final temperature of sphere A is 74 °C, what is the approximate final temperature of sphere B?
(Multiple Choice)
4.8/5  (40)
(40)
Heat is added to a 1.0-kg solid sample of a material at -200 °C. The figure shows the temperature of the material as a function of the heat added. 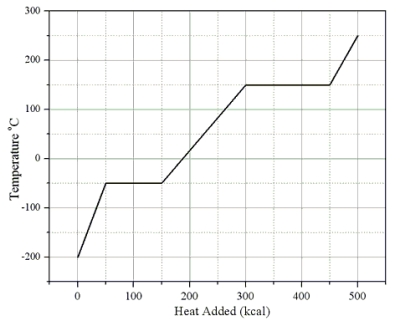 -What is the latent heat of fusion of this material?
-What is the latent heat of fusion of this material?
(Multiple Choice)
4.9/5  (40)
(40)
A tanker ship is filled with 2.25 × 105 m3 of gasoline at a refinery in southern Texas when the temperature is 17.2 °C. When the ship arrives in New York City, the temperature is 1.3 °C. If the coefficient of volumetric expansion for gasoline is 9.50 × 10-4/C°, how much has the volume of the gasoline decreased when it is unloaded in New York?
(Multiple Choice)
4.9/5  (48)
(48)
A 0.0500-kg lead bullet of volume 5.00 × 10–6 m3 at 20.0 °C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At that time, the temperature of the bullet is 327 °C. Use the following information for lead: 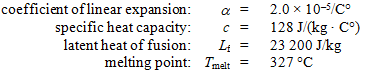 -How much heat was needed to raise the bullet to its final temperature?
-How much heat was needed to raise the bullet to its final temperature?
(Multiple Choice)
4.8/5  (40)
(40)
Zirconium tungstate is an unusual material because its volume shrinks with an increase in temperature for the temperature range 0.3 K to 1050 K (where it decomposes). In fact, the volumetric coefficient of thermal expansion is -26.4 × 10-6/K. Determine the ratio V/V0 for the above mentioned temperature range. Express your answer in percent.
(Multiple Choice)
4.7/5  (34)
(34)
A 0.30-kg lead ball is heated to 90.0 °C and dropped into an ideal calorimeter containing 0.50 kg of water initially at 20.0 °C. What is the final equilibrium temperature of the lead ball? The specific heat capacity of lead is 128 J/(kg · C°); and the specific heat of water is 4186 J/(kg · C°).
(Multiple Choice)
4.9/5  (33)
(33)
Complete the following statement: When solid NH3 passes directly to the gaseous state it is said to
(Multiple Choice)
4.9/5  (37)
(37)
The graph shows the equilibrium vapor pressure versus temperature for a certain liquid and its vapor within an open container. If the container is at sea level, at approximately what temperature will the liquid boil? 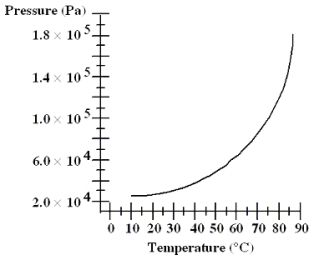
(Multiple Choice)
4.8/5  (32)
(32)
A thin, circular disc is made of lead and has a radius of 0.0350 cm at 20.0 °C. Determine the change in the area of the circle if the temperature is increased to 625.0 °C. The coefficient of linear thermal expansion for lead is 29.0 × 10-6/C°.
(Multiple Choice)
4.9/5  (34)
(34)
An aluminum tank of volume 0.0300 m3 is filled to the top with mercury at 20.0 °C. The tank is placed inside a chamber with an interior temperature of 70.0 °C. The coefficient of volume expansion for mercury is 1.82 × 10-4/C°; and the coefficient of linear expansion of aluminum is 23.0 × 10-6/C°. After the tank and its contents reach thermal equilibrium with the interior of the chamber, how much mercury has spilled?
(Multiple Choice)
4.9/5  (33)
(33)
On a warm summer day, the relative humidity is 30 % when the temperature is 32 °C. Which one of the following statements is true if the temperature suddenly decreases to 26 °C and all other conditions remain the same?
(Multiple Choice)
4.7/5  (38)
(38)
A liquid is in equilibrium with its vapor in a closed vessel. Which one of the following statements is necessarily true?
(Multiple Choice)
4.9/5  (32)
(32)
A gold sphere has a radius of 1.000 cm at 25.0 °C. If 7650 J of heat is added to the sphere, what will the final volume of the sphere be? Gold has a density of 19 300 kg/m3 at 25.0 °C, a specific heat capacity of 129 J/(kg · C°), and a coefficient of volume expansion of 42.0 × 10-6/C°.
(Multiple Choice)
4.8/5  (33)
(33)
Which one of the following statements explains why it is difficult to measure the coefficient of volume expansion for a liquid?
(Multiple Choice)
4.9/5  (35)
(35)
A 2.00-kg metal block slides on a rough, horizontal surface inside an insulated pipe. After sliding a distance of 500.0 m, its temperature is increased by 2.00 °C. Note: Assume that all of the heat generated by frictional heating goes into the metal block. For this metal, the specific heat capacity is 0.150 cal/(g · C°).  -How much work does the force of friction do on the block?
-How much work does the force of friction do on the block?
(Multiple Choice)
4.9/5  (36)
(36)
The units of heat are equivalent to those of which one of the following quantities?
(Multiple Choice)
4.9/5  (39)
(39)
Using the data in the table, determine how many calories are needed to change 100 g of solid X at 10 °C to a vapor at 210 °C. 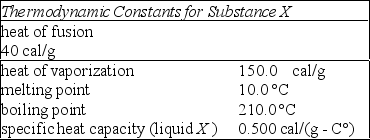
(Multiple Choice)
4.9/5  (38)
(38)
A 2.0-g sample of steam at 100 °C loses 1140 calories of heat. What is the resulting temperature of the sample?
(Multiple Choice)
4.8/5  (39)
(39)
Determine the latent heat of vaporization of unknown substance X in kcal/g if 4.0 g of boiling liquid X are completely vaporized in 1.2 hours by an input of 15 kcal/h into the system by an energy source.
(Multiple Choice)
4.9/5  (26)
(26)
Which one of the following temperatures is approximately equal to the typical temperature of a classroom?
(Multiple Choice)
4.9/5  (36)
(36)
Showing 21 - 40 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)