Exam 12: Temperature and Heat
Exam 1: Introduction and Mathematical Concepts70 Questions
Exam 2: Kinematics in One Dimension103 Questions
Exam 3: Kinematics in Two Dimensions68 Questions
Exam 4: Forces and Newtons Laws of Motion103 Questions
Exam 5: Dynamics of Uniform Circular Motion59 Questions
Exam 6: Work and Energy78 Questions
Exam 7: Impulse and Momentum66 Questions
Exam 8: Rotational Kinematics55 Questions
Exam 9: Rotational Dynamics57 Questions
Exam 10: Simple Harmonic Motion and Elasticity63 Questions
Exam 11: Fluids65 Questions
Exam 12: Temperature and Heat66 Questions
Exam 13: The Transfer of Heat42 Questions
Exam 14: The Ideal Gas Law and Kinetic Theory55 Questions
Exam 15: Thermodynamics79 Questions
Exam 16: Waves and Sound67 Questions
Exam 17: The Principle of Linear Superposition and Interference Phenomena46 Questions
Exam 18: Electric Forces and Electric Fields61 Questions
Exam 19: Electric Potential Energy and the Electric Potential70 Questions
Exam 20: Electric Circuits100 Questions
Exam 21: Magnetic Forces and Magnetic Fields66 Questions
Exam 22: Electromagnetic Induction71 Questions
Exam 23: Alternating Current Circuits84 Questions
Exam 24: Electromagnetic Waves66 Questions
Exam 25: The Refl Ection of Light: Mirrors43 Questions
Exam 26: The Refraction of Light: Lenses and Optical Instruments102 Questions
Exam 27: Interference and the Wave Nature of Light57 Questions
Exam 28: Special Relativity63 Questions
Exam 29: Particles and Waves54 Questions
Exam 30: The Nature of the Atom74 Questions
Exam 31: Nuclear Physics and Radioactivity37 Questions
Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles45 Questions
Select questions type
A soft drink manufacturer claims that a new diet drink is "low Joule." The label indicates the available energy per serving is 6300 J. What is the equivalent of this energy in Calories (1 Calorie = 1000 cal)?
(Multiple Choice)
4.8/5  (40)
(40)
A 200.0-kg object is attached via an ideal pulley system to paddle wheels that are submerged in 0.480 kg of glycerin at 20.0 °C in an insulated container as shown. Then, the object falls through a distance of 5.00 m causing the paddle wheel to turn. Assuming all of the mechanical energy lost by the falling object goes into the water, determine the final temperature of the glycerin. The specific heat capacity of glycerin is 2410 J/  Co).
Co). 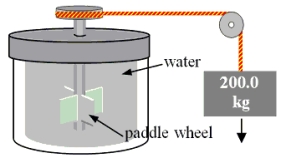
(Multiple Choice)
5.0/5  (33)
(33)
Complete the following statement: When a substance undergoes fusion it
(Multiple Choice)
4.9/5  (27)
(27)
The coefficient of linear expansion of steel is 12 × 10-6/C°. A railroad track is made of individual rails of steel 1.0 km in length. By what length would these rails change between a cold day when the temperature is -10 °C and a hot day at 30 °C?
(Multiple Choice)
5.0/5  (39)
(39)
Ryan places 0.150 kg of boiling water in a thermos bottle. How many kilograms of ice at -12.0 °C must Ryan add to the thermos so that the equilibrium temperature of the water is 75.0 °C?
(Multiple Choice)
4.9/5  (37)
(37)
A 2.00-kg metal block slides on a rough, horizontal surface inside an insulated pipe. After sliding a distance of 500.0 m, its temperature is increased by 2.00 °C. Note: Assume that all of the heat generated by frictional heating goes into the metal block. For this metal, the specific heat capacity is 0.150 cal/(g · C°).  -What is the coefficient of sliding friction between the block and the surface?
-What is the coefficient of sliding friction between the block and the surface?
(Multiple Choice)
4.9/5  (33)
(33)
Showing 61 - 66 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)