Exam 19: The First Law of Thermodynamics
Exam 1: Units,physical Quantities,and Vectors107 Questions
Exam 2: Motion Along a Straight Line59 Questions
Exam 3: Motion in Two or Three Dimensions50 Questions
Exam 4: Newtons Laws of Motion44 Questions
Exam 5: Applying Newtons Laws95 Questions
Exam 6: Work and Kinetic Energy54 Questions
Exam 7: Potential Energy and Energy Conservation55 Questions
Exam 8: Momentum,impulse,and Collisions50 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Dynamics of Rotational Motion49 Questions
Exam 11: Equilibrium and Elasticity50 Questions
Exam 12: Fluid Mechanics50 Questions
Exam 13: Gravitation50 Questions
Exam 14: Periodic Motion50 Questions
Exam 15: Mechanical Waves44 Questions
Exam 16: Sound and Hearing65 Questions
Exam 17: Temperature and Heat63 Questions
Exam 18: Thermal Properties of Matter58 Questions
Exam 19: The First Law of Thermodynamics52 Questions
Exam 20: The Second Law of Thermodynamics50 Questions
Exam 21: Electric Charge and Electric Field60 Questions
Exam 22: Gausss Law41 Questions
Exam 23: Electric Potential55 Questions
Exam 24: Capacitance and Dielectrics52 Questions
Exam 25: Current,resistance,and Electromotive Force55 Questions
Exam 26: Direct-Current Circuits53 Questions
Exam 27: Magnetic Field and Magnetic Forces42 Questions
Exam 28: Sources of Magnetic Field52 Questions
Exam 29: Electromagnetic Induction39 Questions
Exam 30: Inductance27 Questions
Exam 31: Alternating Current50 Questions
Exam 32: Electromagnetic Waves47 Questions
Exam 33: The Nature and Propagation of Light28 Questions
Exam 34: Geometric Optics81 Questions
Exam 35: Interference33 Questions
Exam 36: Diffraction49 Questions
Exam 37: Relativity51 Questions
Exam 38: Photons: Light Waves Behaving As Particles38 Questions
Exam 39: Particles Behaving As Waves52 Questions
Exam 40: Quantum Mechanics43 Questions
Exam 41: Atomic Structure53 Questions
Exam 42: Molecules and Condensed Matter31 Questions
Exam 43: Nuclear Physics90 Questions
Exam 44: Particle Physics and Cosmology54 Questions
Select questions type
An ideal gas with γ = 1.30 occupies 7.0 L at 300 K and 200 kPa pressure.It is compressed adiabatically to 1/7 of its original volume,then cooled at constant volume to 300 K,and finally allowed to expand isothermally to 7.0 L.How much work does the gas do during this process? The ideal gas constant is R = 8.314 J/mol • K = 0.0821 L • atm/mol • K.
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
A
When a fixed amount of ideal gas goes through an isothermal expansion,
Free
(Multiple Choice)
4.8/5  (44)
(44)
Correct Answer:
A
A container of ideal gas has a movable frictionless piston.This container is placed in a very large water bath and slowly compressed so that the temperature of the gas remains constant and equal to the temperature of the water.Which of the following statements about this gas are true for this process? (There may be more than one correct choice.)
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
A,C
An expansion process on an ideal diatomic gas has a linear path between the initial and final states on a pV diagram.The initial pressure is 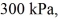 the initial volume is
the initial volume is 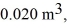 and the initial temperature is
and the initial temperature is 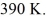 The final pressure is
The final pressure is 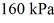 and the final temperature is
and the final temperature is 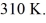 The change in the internal (thermal)energy of the gas is closest to
The change in the internal (thermal)energy of the gas is closest to
(Multiple Choice)
4.9/5  (32)
(32)
During an adiabatic process,20 moles of a monatomic ideal gas undergo a temperature change from 450 K to 320 K starting from an initial pressure is 400 kPa.The ideal gas constant is 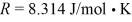 .
(a)What is the final volume of the gas?
(b)How much heat does the gas exchange during this process?
(c)What is the change in the internal (thermal)energy of the gas during this process?
.
(a)What is the final volume of the gas?
(b)How much heat does the gas exchange during this process?
(c)What is the change in the internal (thermal)energy of the gas during this process?
(Short Answer)
4.7/5  (28)
(28)
The temperature of an ideal gas in a sealed 0.40- 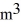 rigid container is reduced from 350 K to
rigid container is reduced from 350 K to 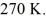 The final pressure of the gas is
The final pressure of the gas is 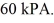 The molar heat capacity at constant volume of the gas is 28.0 J/mol • K.The heat absorbed by the gas is closest to
The molar heat capacity at constant volume of the gas is 28.0 J/mol • K.The heat absorbed by the gas is closest to
(Multiple Choice)
4.8/5  (33)
(33)
An ideal gas with γ = 1.67 is initially at 0°C in a volume of 10.0 L at a pressure of 1.00 atm.It is then expanded adiabatically to a volume of 10.4 L.What is the final temperature of the gas?
(Multiple Choice)
4.8/5  (31)
(31)
A monatomic ideal gas undergoes an isothermal expansion at 300 K,as the volume increased from 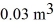 to
to 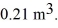 The final pressure of the gas is
The final pressure of the gas is 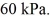 The ideal gas constant is
The ideal gas constant is 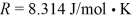 .The change in the internal (thermal)energy of the gas is closest to
.The change in the internal (thermal)energy of the gas is closest to
(Multiple Choice)
4.8/5  (31)
(31)
The figure shows a pV diagram for 4.3 g of oxygen gas (O2)in a sealed container.The temperature T1 of the gas in state 1 is 21°C.What are the temperatures T3 and T4 of the gas in states 3 and 4? The ideal gas constant is R = 8.314 J/mol • K = 0.0821 L ∙ atm/mol • K,and the ATOMIC weight of oxygen is 16 g/mol. 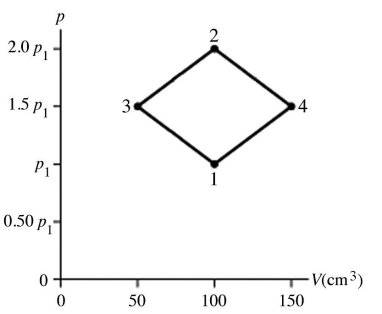
(Multiple Choice)
4.9/5  (34)
(34)
The gas in a perfectly insulated system does work at a rate of 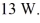 At what rate is the internal (thermal)energy of the gas changing?
At what rate is the internal (thermal)energy of the gas changing?
(Multiple Choice)
4.8/5  (33)
(33)
A system has a heat source supplying heat to an ideal gas at a rate of 187.0 W and the gas is doing work at a rate of 130.9 W.At what rate is the internal (thermal)energy of the gas changing?
(Multiple Choice)
4.8/5  (26)
(26)
A compression,at a constant pressure of 190 kPa,is performed on 5.0 moles of an ideal monatomic gas.The compression reduces the volume of the gas from 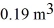 to
to 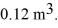 The ideal gas constant is R = 8.314 J/mol • K. The work done by the gas is closest to
The ideal gas constant is R = 8.314 J/mol • K. The work done by the gas is closest to
(Multiple Choice)
4.8/5  (36)
(36)
A fixed amount of ideal gas goes through a process abc.In state a,the temperature of the gas is 152°C,its pressure is 1.25 atm,and it occupies a volume of 0.250 m3.It then undergoes an isothermal expansion to state b that doubles its volume,followed by an isobaric compression back to its original volume at state c.(Hint: First show this process on a pV diagram.)The ideal gas constant is 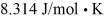 ,and
,and 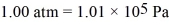 .
(a)How many moles does this gas contain?
(b)What is the change in the internal energy of the gas between states a and b?
(c)What is the net work done on (or by)this gas during the entire process?
(d)What is the temperature of the gas in state c?
.
(a)How many moles does this gas contain?
(b)What is the change in the internal energy of the gas between states a and b?
(c)What is the net work done on (or by)this gas during the entire process?
(d)What is the temperature of the gas in state c?
(Short Answer)
4.7/5  (36)
(36)
During an isothermal process,5.0 J of heat is removed from an ideal gas.How much work does the gas do during this process?
(Multiple Choice)
4.8/5  (29)
(29)
When a fixed amount of ideal gas goes through an adiabatic expansion,
(Multiple Choice)
4.9/5  (43)
(43)
In a thermodynamic process involving 7.8 moles of an ideal gas,the gas is at an initial temperature of 24°C and has an initial volume of 0.040 m3.The gas expands adiabatically to a volume of 0.080 m3.For this gas,CV = 12.27 J/mol • K,and the ideal gas constant is 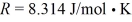 .Calculate the work done by the gas during this expansion.
.Calculate the work done by the gas during this expansion.
(Short Answer)
4.9/5  (43)
(43)
A monatomic ideal gas undergoes an isothermal expansion at 300 K,as the volume increased from 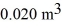 to
to 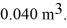 The final pressure is
The final pressure is 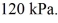 The ideal gas constant is
The ideal gas constant is 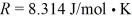 .The heat transfer to the gas is closest to
.The heat transfer to the gas is closest to
(Multiple Choice)
4.7/5  (35)
(35)
An ideal gas increases in temperature from 22°C to 42°C by two different processes.In one process,the temperature increases at constant volume,and in the other process the temperature increases at constant pressure.Which of the following statements about this gas are correct? (There may be more than one correct choice.)
(Multiple Choice)
5.0/5  (41)
(41)
3.0 moles of an ideal gas with a molar heat capacity at constant volume of 4.9 cal/(mol • K)and a molar heat capacity at constant pressure of 6.9 cal/(mol • K)starts at 300 K and is heated at constant pressure to 320 K,then cooled at constant volume to its original temperature.How much heat flows into the gas during this two-step process?
(Multiple Choice)
4.9/5  (33)
(33)
A cylinder contains 1.2 moles of ideal gas,initially at a temperature of 116°C.The cylinder is provided with a frictionless piston,which maintains a constant pressure of 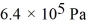 on the gas.The gas is cooled until its temperature has decreased to
on the gas.The gas is cooled until its temperature has decreased to 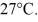 For the gas
For the gas 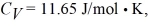 and the ideal gas constant
and the ideal gas constant 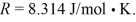 (a)Find the work done by (or on)the gas during this process.Is the work done by or on the gas?
(b)What is the change in the internal (thermal)energy of the gas during this process?
(c)How much heat is transferred to (or from)the gas during this process? Does this heat flow into or out of the gas?
(a)Find the work done by (or on)the gas during this process.Is the work done by or on the gas?
(b)What is the change in the internal (thermal)energy of the gas during this process?
(c)How much heat is transferred to (or from)the gas during this process? Does this heat flow into or out of the gas?
(Essay)
4.8/5  (25)
(25)
Showing 1 - 20 of 52
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)