Exam 43: Nuclear Physics
Exam 1: Units,physical Quantities,and Vectors107 Questions
Exam 2: Motion Along a Straight Line59 Questions
Exam 3: Motion in Two or Three Dimensions50 Questions
Exam 4: Newtons Laws of Motion44 Questions
Exam 5: Applying Newtons Laws95 Questions
Exam 6: Work and Kinetic Energy54 Questions
Exam 7: Potential Energy and Energy Conservation55 Questions
Exam 8: Momentum,impulse,and Collisions50 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Dynamics of Rotational Motion49 Questions
Exam 11: Equilibrium and Elasticity50 Questions
Exam 12: Fluid Mechanics50 Questions
Exam 13: Gravitation50 Questions
Exam 14: Periodic Motion50 Questions
Exam 15: Mechanical Waves44 Questions
Exam 16: Sound and Hearing65 Questions
Exam 17: Temperature and Heat63 Questions
Exam 18: Thermal Properties of Matter58 Questions
Exam 19: The First Law of Thermodynamics52 Questions
Exam 20: The Second Law of Thermodynamics50 Questions
Exam 21: Electric Charge and Electric Field60 Questions
Exam 22: Gausss Law41 Questions
Exam 23: Electric Potential55 Questions
Exam 24: Capacitance and Dielectrics52 Questions
Exam 25: Current,resistance,and Electromotive Force55 Questions
Exam 26: Direct-Current Circuits53 Questions
Exam 27: Magnetic Field and Magnetic Forces42 Questions
Exam 28: Sources of Magnetic Field52 Questions
Exam 29: Electromagnetic Induction39 Questions
Exam 30: Inductance27 Questions
Exam 31: Alternating Current50 Questions
Exam 32: Electromagnetic Waves47 Questions
Exam 33: The Nature and Propagation of Light28 Questions
Exam 34: Geometric Optics81 Questions
Exam 35: Interference33 Questions
Exam 36: Diffraction49 Questions
Exam 37: Relativity51 Questions
Exam 38: Photons: Light Waves Behaving As Particles38 Questions
Exam 39: Particles Behaving As Waves52 Questions
Exam 40: Quantum Mechanics43 Questions
Exam 41: Atomic Structure53 Questions
Exam 42: Molecules and Condensed Matter31 Questions
Exam 43: Nuclear Physics90 Questions
Exam 44: Particle Physics and Cosmology54 Questions
Select questions type
The material used in certain nuclear bombs is 239Pu,which has a half-life of about 20,000 years.How long must we wait for a buried stockpile of this substance to decay to 4.0% of its original 239Pu mass?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
A
The iron nucleus has the greatest binding energy of any nucleus.
Free
(True/False)
4.9/5  (35)
(35)
Correct Answer:
False
The stability of  Fe with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known:
Fe with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known:  He: 4.002603 u
He: 4.002603 u  Cr: 51.944768 u
Cr: 51.944768 u  Mn: 55.938907 u
Mn: 55.938907 u 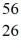 Fe: 55.934939 u
Fe: 55.934939 u 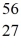 Co: 55.939841 u The
Co: 55.939841 u The 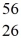 Fe nuclide is
Fe nuclide is
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
A
What mass of 14C (having a half-life of 5730 years)do you need to provide a decay rate of 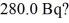 (1 u = 1.6605 × 10-27 kg)
(1 u = 1.6605 × 10-27 kg)
(Multiple Choice)
4.8/5  (32)
(32)
An excited 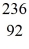 U* nucleus undergoes fission into two fragments,as shown:
U* nucleus undergoes fission into two fragments,as shown: 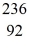 U* →
U* → 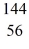 Ba +
Ba + 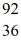 Kr The following atomic masses are known:
Kr The following atomic masses are known: 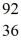 Kr: 91.926270 u
Kr: 91.926270 u 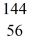 Ba: 143.922845 u
Ba: 143.922845 u 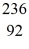 U*: 236.045563 u
Assume,at a given instant,that the two fission fragments are spherical,just barely in contact,and carry spherically symmetric charge distributions.At that instant,what is the electrostatic interaction energy of the two fragments,in MeV? (1 u = 1.6605 × 10-27 kg = 9
U*: 236.045563 u
Assume,at a given instant,that the two fission fragments are spherical,just barely in contact,and carry spherically symmetric charge distributions.At that instant,what is the electrostatic interaction energy of the two fragments,in MeV? (1 u = 1.6605 × 10-27 kg = 9 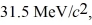
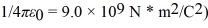
(Multiple Choice)
4.8/5  (28)
(28)
Consider two different isotopes of the same neutral element.Which statements about these isotopes are true? (There may be more than one correct choice.)
(Multiple Choice)
4.8/5  (29)
(29)
Calculate the amount of energy that is released in the fusion reaction 2H + 2H → 4He,given the masses: 2H: 2.014102 u
4He: 4.002603 u
(1 u = 931.5 MeV/c2)
(Multiple Choice)
4.8/5  (38)
(38)
The maximum permissible workday dose for occupational exposure to radiation is 26 mrem.A 55-kg laboratory technician absorbs 3.3 mJ of 0.40-MeV gamma rays in a workday.The relative biological efficiency (RBE)for gamma rays is 1.00.What is the ratio of the equivalent dosage received by the technician to the maximum permissible equivalent dosage?
(Multiple Choice)
4.9/5  (43)
(43)
In massive stars,three helium nuclei fuse together,forming a carbon nucleus.This reaction heats the core of the star.The net mass of the three helium nuclei must therefore be
(Multiple Choice)
5.0/5  (43)
(43)
For a 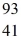 Nb atom,the number of protons,neutrons,and electrons in the atom is
Nb atom,the number of protons,neutrons,and electrons in the atom is
(Multiple Choice)
4.8/5  (34)
(34)
The primary source of the energy radiated by a star,such as the sun,is
(Multiple Choice)
4.8/5  (42)
(42)
Today,the uranium found on Earth contains 0.720% 235U (with a half-life of 0.700 billion years)and 99.28% 238U (with a half-life of 4.50 billion years).At a time 2.20 billion years ago,what percent of the uranium on Earth was 238U (assuming that no other uranium isotopes were present)?
(Multiple Choice)
4.9/5  (43)
(43)
The neutral deuterium atom,  H,has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.What is the binding energy of the
H,has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.What is the binding energy of the  H nucleus?
H nucleus? 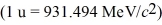
(Multiple Choice)
4.7/5  (43)
(43)
The radioactive nuclei 60Co is widely used in medical applications.It undergoes beta decay,and the total energy of the decay process is 2.82 MeV per decay event.The half-life of this nucleus is 272 days.Suppose that a patient is given a dose of 6.9 µCi of 60Co.If all of this material decayed while in the patient's body,what would be the total energy deposited there? 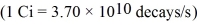
(Multiple Choice)
4.9/5  (34)
(34)
The reaction 2H + 2H →? 3H + 1H releases 4.03 MeV of energy.If 1.0 kg of deuterium were to go through this reaction,how much energy would be produced? 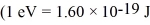 ,
, 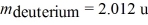 ,1 u = 931.5 MeV/c2 = 1.6605 × 10-27 kg)
,1 u = 931.5 MeV/c2 = 1.6605 × 10-27 kg)
(Multiple Choice)
4.8/5  (36)
(36)
Consider the short-lived neutral isotope represented by 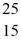 X.Which of the following statements about this isotope are correct? (There may be more than one correct choice.)
X.Which of the following statements about this isotope are correct? (There may be more than one correct choice.)
(Multiple Choice)
4.8/5  (32)
(32)
What is the binding energy per nucleon for 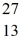 Al? The neutral
Al? The neutral 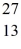 Al atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.(1 u = 931.494 MeV/c2)
Al atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.(1 u = 931.494 MeV/c2)
(Multiple Choice)
4.9/5  (24)
(24)
Plutonium-239 decays into uranium-235 plus an alpha particle.The energy released in the process is 5.24 MeV.Given the following mass values 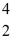 He: 4.002603 u
He: 4.002603 u 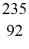 U: 235.043924 u what is the mass of
U: 235.043924 u what is the mass of 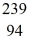 Pu in atomic mass units? (1 u = 931.494 MeV/c2)
Pu in atomic mass units? (1 u = 931.494 MeV/c2)
(Multiple Choice)
4.8/5  (34)
(34)
A radioactive nuclide of atomic number Z emits an electron,then the daughter nuclide emits a gamma ray.What is the atomic number of the resulting nuclide after both processes?
(Multiple Choice)
4.9/5  (33)
(33)
Going from medium mass nuclei to heavy nuclei,the average binding energy per nucleon
(Multiple Choice)
4.8/5  (33)
(33)
Showing 1 - 20 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)