Exam 41: Atomic Structure
Exam 1: Units,physical Quantities,and Vectors107 Questions
Exam 2: Motion Along a Straight Line59 Questions
Exam 3: Motion in Two or Three Dimensions50 Questions
Exam 4: Newtons Laws of Motion44 Questions
Exam 5: Applying Newtons Laws95 Questions
Exam 6: Work and Kinetic Energy54 Questions
Exam 7: Potential Energy and Energy Conservation55 Questions
Exam 8: Momentum,impulse,and Collisions50 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Dynamics of Rotational Motion49 Questions
Exam 11: Equilibrium and Elasticity50 Questions
Exam 12: Fluid Mechanics50 Questions
Exam 13: Gravitation50 Questions
Exam 14: Periodic Motion50 Questions
Exam 15: Mechanical Waves44 Questions
Exam 16: Sound and Hearing65 Questions
Exam 17: Temperature and Heat63 Questions
Exam 18: Thermal Properties of Matter58 Questions
Exam 19: The First Law of Thermodynamics52 Questions
Exam 20: The Second Law of Thermodynamics50 Questions
Exam 21: Electric Charge and Electric Field60 Questions
Exam 22: Gausss Law41 Questions
Exam 23: Electric Potential55 Questions
Exam 24: Capacitance and Dielectrics52 Questions
Exam 25: Current,resistance,and Electromotive Force55 Questions
Exam 26: Direct-Current Circuits53 Questions
Exam 27: Magnetic Field and Magnetic Forces42 Questions
Exam 28: Sources of Magnetic Field52 Questions
Exam 29: Electromagnetic Induction39 Questions
Exam 30: Inductance27 Questions
Exam 31: Alternating Current50 Questions
Exam 32: Electromagnetic Waves47 Questions
Exam 33: The Nature and Propagation of Light28 Questions
Exam 34: Geometric Optics81 Questions
Exam 35: Interference33 Questions
Exam 36: Diffraction49 Questions
Exam 37: Relativity51 Questions
Exam 38: Photons: Light Waves Behaving As Particles38 Questions
Exam 39: Particles Behaving As Waves52 Questions
Exam 40: Quantum Mechanics43 Questions
Exam 41: Atomic Structure53 Questions
Exam 42: Molecules and Condensed Matter31 Questions
Exam 43: Nuclear Physics90 Questions
Exam 44: Particle Physics and Cosmology54 Questions
Select questions type
What is the energy of an incident photon that is just enough to excite a hydrogen atom from its ground state to its n = 4 state?
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
A
Two entangled fermions each have equal probabilities of being in one of two states (state 1 or state 2).The fermions are separated so that no forces act between them.One of the fermions is then studied and found to be in state 1.What is the probability that,when studied,the second particle will also be found in state 1?
Free
(Multiple Choice)
4.9/5  (40)
(40)
Correct Answer:
A
An atom has completely filled inner shells and a single valence electron in an excited p state.The filled inner shells have an orbital momentum equal to zero.A magnetic field is applied,defining the z-axis along the field.Which of the following sets of angles are possible angles between the magnetic field and the orbital angular momentum?
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
E
What is the greatest total angular momentum J for an electron in the n = 2 shell?
(Multiple Choice)
4.9/5  (37)
(37)
An electron initially in a 4p state decays to a lower energy state.Which energy state is forbidden?
(Multiple Choice)
4.8/5  (31)
(31)
An alkali metal atom is in the ground state.The orbital angular momentum equals zero and the spin angular momentum is entirely due to the single valence electron.A magnetic field is applied that splits the ground state energy level into two levels,27 μeV apart.What is the strength of the applied magnetic field? (h = 6.626 × 10-34 J ∙ s,Bohr magneton = μB = 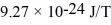 ,1 eV = 1.60 × 10-19 J)
,1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.9/5  (34)
(34)
A neutral atom has an electron configuration of 1s22s22p63s23p2.How many protons does it have in its nucleus?
(Multiple Choice)
4.8/5  (26)
(26)
An atom has completely filled inner shells and a single valence electron in an excited p state.The filled inner shells have an orbital momentum equal to zero.What is the magnitude of the orbital angular momentum L of the atom?
(Multiple Choice)
4.9/5  (31)
(31)
Model a hydrogen atom as a three-dimensional potential well with U0 = 0 in the region 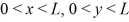 ,and 0 < z < L,and infinite otherwise,with L = 1.0 × 10-10 m.Which of the following is NOT one of the lowest three energy levels of an electron in this model?
,and 0 < z < L,and infinite otherwise,with L = 1.0 × 10-10 m.Which of the following is NOT one of the lowest three energy levels of an electron in this model?
(Multiple Choice)
4.8/5  (32)
(32)
The energy of an electron in the p-level of an atom is changed in the presence of a magnetic field of magnitude 4.6 T.What is the difference between the largest and smallest possible energies? (Bohr magneton = μB = 9.27 × 10-24 J/T)
(Short Answer)
4.8/5  (39)
(39)
How fast must a hydrogen atom be traveling for its kinetic energy to be just enough to excite the ground-state atom to its first excited state in a collision? (1 eV = 1.60 × 10-19 J, 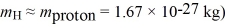
(Multiple Choice)
4.8/5  (42)
(42)
The normalized wave function for a hydrogen atom in the 1s state is given by 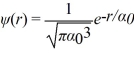 where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
where α0 is the Bohr radius,which is equal to 5.29 × 10-11 m.What is the probability of finding the electron at a distance greater than 7.8 α0 from the proton?
(Multiple Choice)
4.9/5  (32)
(32)
The magnitude of the orbital angular momentum L of an electron in a certain atom is equal to 3.464ħ.Which of the following angles could NOT be the angle between the orbital angular momentum vector of the electron and an arbitrary z-direction?
(Multiple Choice)
4.7/5  (26)
(26)
If two electrons in an atom have the same energy,then they must have the same four quantum numbers.
(True/False)
4.7/5  (35)
(35)
The only INVALID electron state and shell designation among the following is
(Multiple Choice)
4.9/5  (34)
(34)
The binding energy of the hydrogen atom in its ground state is -13.6 eV.What is the energy when it is in the n = 5 state?
(Multiple Choice)
4.9/5  (32)
(32)
An alkali metal atom is in the ground state.The orbital angular momentum equals zero and the spin angular momentum is entirely due to the single valence electron.A magnetic field is applied that splits the ground state energy level into two levels,65 μeV apart.A photon,absorbed by the atom,induces a transition between the two levels.What is the wavelength of the photon? (c = 3.00 × 108 m/s,h = 6.626 × 10-34 J • s,Bohr magneton = μB = 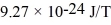 ,
, 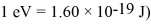
(Multiple Choice)
4.9/5  (32)
(32)
What is the electron configuration for ground state Li,which has 3 electrons?
(Multiple Choice)
4.8/5  (31)
(31)
An s state (l = 0)energy level is split into two levels by an applied magnetic field.A photon of microwave radiation having frequency 60 GHz induces a transition between the two levels.What is the magnitude of the applied magnetic field? (h = 6.626 × 10-34 J • s, 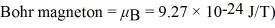
(Multiple Choice)
4.7/5  (38)
(38)
Showing 1 - 20 of 53
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)