Exam 18: Thermal Properties of Matter
Exam 1: Units,physical Quantities,and Vectors107 Questions
Exam 2: Motion Along a Straight Line59 Questions
Exam 3: Motion in Two or Three Dimensions50 Questions
Exam 4: Newtons Laws of Motion44 Questions
Exam 5: Applying Newtons Laws95 Questions
Exam 6: Work and Kinetic Energy54 Questions
Exam 7: Potential Energy and Energy Conservation55 Questions
Exam 8: Momentum,impulse,and Collisions50 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Dynamics of Rotational Motion49 Questions
Exam 11: Equilibrium and Elasticity50 Questions
Exam 12: Fluid Mechanics50 Questions
Exam 13: Gravitation50 Questions
Exam 14: Periodic Motion50 Questions
Exam 15: Mechanical Waves44 Questions
Exam 16: Sound and Hearing65 Questions
Exam 17: Temperature and Heat63 Questions
Exam 18: Thermal Properties of Matter58 Questions
Exam 19: The First Law of Thermodynamics52 Questions
Exam 20: The Second Law of Thermodynamics50 Questions
Exam 21: Electric Charge and Electric Field60 Questions
Exam 22: Gausss Law41 Questions
Exam 23: Electric Potential55 Questions
Exam 24: Capacitance and Dielectrics52 Questions
Exam 25: Current,resistance,and Electromotive Force55 Questions
Exam 26: Direct-Current Circuits53 Questions
Exam 27: Magnetic Field and Magnetic Forces42 Questions
Exam 28: Sources of Magnetic Field52 Questions
Exam 29: Electromagnetic Induction39 Questions
Exam 30: Inductance27 Questions
Exam 31: Alternating Current50 Questions
Exam 32: Electromagnetic Waves47 Questions
Exam 33: The Nature and Propagation of Light28 Questions
Exam 34: Geometric Optics81 Questions
Exam 35: Interference33 Questions
Exam 36: Diffraction49 Questions
Exam 37: Relativity51 Questions
Exam 38: Photons: Light Waves Behaving As Particles38 Questions
Exam 39: Particles Behaving As Waves52 Questions
Exam 40: Quantum Mechanics43 Questions
Exam 41: Atomic Structure53 Questions
Exam 42: Molecules and Condensed Matter31 Questions
Exam 43: Nuclear Physics90 Questions
Exam 44: Particle Physics and Cosmology54 Questions
Select questions type
At what temperature would the root-mean-square speed (thermal speed)of oxygen molecules be 13.0 m/s? Assume that oxygen approximates an ideal gas.The mass of one O2 molecule is 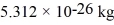 .The Boltzmann constant is
.The Boltzmann constant is 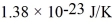 .
.
Free
(Multiple Choice)
4.9/5  (35)
(35)
Correct Answer:
A
If a certain sample of an ideal gas has a temperature of 109°C and exerts a pressure of 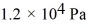 on the walls of its container,how many gas molecules are present in each cubic centimeter of volume? The ideal gas constant is 8.314 J/mol • K and Avogadro's number is 6.022 × 1023 molecules/mol.
on the walls of its container,how many gas molecules are present in each cubic centimeter of volume? The ideal gas constant is 8.314 J/mol • K and Avogadro's number is 6.022 × 1023 molecules/mol.
Free
(Short Answer)
4.9/5  (37)
(37)
Correct Answer:
2.3 × 1018 molecules
An ideal gas is kept in a rigid container.When its temperature is 100 K,the mean-free-path of the gas molecules is λ.What will be the mean-free-path of the molecules at 400 K?
Free
(Multiple Choice)
4.7/5  (42)
(42)
Correct Answer:
C
An ideal gas is at a pressure 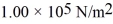 and occupies a volume 2.00 m3.If the gas is compressed to a volume 1.00 m3 while the temperature remains constant,what will be the new pressure in the gas?
and occupies a volume 2.00 m3.If the gas is compressed to a volume 1.00 m3 while the temperature remains constant,what will be the new pressure in the gas?
(Multiple Choice)
4.9/5  (37)
(37)
What is the total translational kinetic energy in a test chamber filled with nitrogen (N2)at 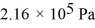 and
and 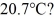 The dimensions of the chamber are
The dimensions of the chamber are 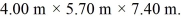 The ATOMIC weight of nitrogen is 28.0 g/mol,Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K.
The ATOMIC weight of nitrogen is 28.0 g/mol,Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K.
(Short Answer)
4.7/5  (40)
(40)
What is the mean free path for the molecules in an ideal gas when the pressure is 100 kPa and the temperature is 300 K given that the collision cross-section for the molecules of that gas is 2.0 × 10-20 m2? The Boltzmann constant is 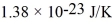 ,Avogadro's number is
,Avogadro's number is 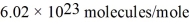 ,and the ideal gas constant is
,and the ideal gas constant is 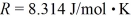 =
= 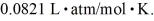
(Multiple Choice)
4.7/5  (37)
(37)
An oxygen molecule falls in a vacuum.From what height must it fall so that its kinetic energy at the bottom equals the average energy of an oxygen molecule at 800 K? (The Boltzmann constant is 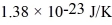 ,the molecular weight of oxygen is 32.0 g/mol,and Avogadro's number is
,the molecular weight of oxygen is 32.0 g/mol,and Avogadro's number is 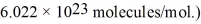
(Multiple Choice)
4.9/5  (34)
(34)
How many moles of water (H2O)molecules are in a 4.00 m3 container at a pressure 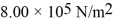 and temperature 600°C? The ideal gas constant is
and temperature 600°C? The ideal gas constant is 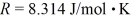 =
= 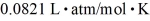 .
.
(Multiple Choice)
4.9/5  (39)
(39)
On a hot summer day,the temperature is 40.0°C and the pressure is 1.01 × 105 Pa.Let us model the air as all nitrogen of molecular mass 28.0 g/mol having molecules of diameter 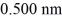 that are moving at their root-mean-square speed.Avogadro's number is
that are moving at their root-mean-square speed.Avogadro's number is 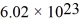 molecules/mol,the ideal gas constant is 8.31 J/mol • K,and the Boltzmann constant is
molecules/mol,the ideal gas constant is 8.31 J/mol • K,and the Boltzmann constant is 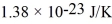 .Calculate reasonable estimates for
(a)the root-mean-square speed of the nitrogen molecules.
(b)the average distance a typical molecule travels between collisions.
(c)the average time a molecule travels between collisions,assuming that the molecules are moving at their root-mean-square speeds.
(d)the number of collisions an average molecule undergoes per second.
.Calculate reasonable estimates for
(a)the root-mean-square speed of the nitrogen molecules.
(b)the average distance a typical molecule travels between collisions.
(c)the average time a molecule travels between collisions,assuming that the molecules are moving at their root-mean-square speeds.
(d)the number of collisions an average molecule undergoes per second.
(Short Answer)
4.9/5  (23)
(23)
The interior of a refrigerator has a volume of 0.600 m3.The temperature inside the refrigerator in 282 K,and the pressure is 101 kPa.If the molecular weight of air is 29 g/mol,what is the mass of air inside the refrigerator? The ideal gas constant is R = 8.314 J/mol • K = 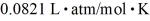 .
.
(Multiple Choice)
4.9/5  (28)
(28)
Which contains more moles of material: 80 grams of helium gas (He,having atomic weight 4.0 g/mol)or 400 grams of argon gas (Ar,having atomic weight 40 g/mol)?
(Multiple Choice)
4.8/5  (30)
(30)
For a fixed amount of gas,if the absolute temperature of the gas is doubled,what happens to the pressure of the gas?
(Multiple Choice)
4.8/5  (32)
(32)
The root-mean-square speed (thermal speed)for a certain gas at 100°C is 0.500 km/s.If the temperature of the gas is now increased to 200°C,the root-mean-square (thermal)speed will be closest to
(Multiple Choice)
4.9/5  (37)
(37)
The mean free path of an oxygen molecule is 2.0 × 10-5 m,when the gas is at a pressure of 120 Pa and a temperature of 275 K.The atomic mass of oxygen is 16.0 g/mol,the Boltzmann constant is 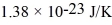 ,Avogadro's number is
,Avogadro's number is 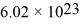 molecules/mole,and the ideal gas constant is
molecules/mole,and the ideal gas constant is 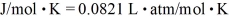 .The radius of an oxygen molecule is closest to
.The radius of an oxygen molecule is closest to
(Multiple Choice)
4.9/5  (30)
(30)
2.0 L of a ideal nitrogen gas (N2)are at 0.00°C and 1.0 atm.The ideal gas constant is 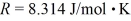 = 0.0821 L • atm/mol • K,Avogadro's number is 6.022 × 1023 molecules/mol,and the ATOMIC mass of nitrogen is 14 g/mol.
(a)Determine the number of moles of N2.
(b)How many molecules of N2 are present?
(c)What is the mass of this gas?
= 0.0821 L • atm/mol • K,Avogadro's number is 6.022 × 1023 molecules/mol,and the ATOMIC mass of nitrogen is 14 g/mol.
(a)Determine the number of moles of N2.
(b)How many molecules of N2 are present?
(c)What is the mass of this gas?
(Short Answer)
4.7/5  (34)
(34)
A bag of potato chips contains 2.00 L of air when it is sealed at sea level at a pressure of 1.00 atm and a temperature of 20.0°C.What will be the volume of the air in the bag if you take it with you,still sealed,to the mountains where the temperature is 7.00°C and atmospheric pressure is 70.0 kPa? Assume that the bag behaves like a balloon and that the air in the bag is in thermal equilibrium with the outside air.(1 atm = 1.01 × 105 Pa)
(Multiple Choice)
4.8/5  (36)
(36)
What is the mean free path of molecules in an ideal gas in which the mean collision time is 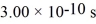 ,the temperature is 300 K,and the mass of the molecules is
,the temperature is 300 K,and the mass of the molecules is 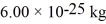 ? Assume that the molecules are moving at their root-mean-square speeds.The Boltzmann constant is
? Assume that the molecules are moving at their root-mean-square speeds.The Boltzmann constant is 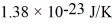 .
.
(Multiple Choice)
4.8/5  (40)
(40)
A 3.2-L volume of neon gas (Ne)is at a pressure of 3.3 atm and a temperature of 330 K.The atomic mass of neon is 20.2 g/mol,Avogadro's number is 6.022 × 1023 molecules/mol,and the ideal gas constant is 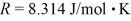 =
= 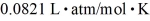 .The mass of the neon gas is closest to
.The mass of the neon gas is closest to
(Multiple Choice)
4.8/5  (33)
(33)
3.00 moles of an ideal gas at a pressure of 250 kPa are held in a container of volume of 25.0 L.The ideal gas constant is R = 8.314 J/mol • K = 0.0821 L ∙ atm/mol • K,and 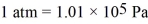 .The temperature of this gas is closest to
.The temperature of this gas is closest to
(Multiple Choice)
4.7/5  (42)
(42)
A cubic box with sides of 20.0 cm contains 2.00 × 1023 molecules of helium with a root-mean-square speed (thermal speed)of 200 m/s.The mass of a helium molecule is 3.40 × 10-27 kg.What is the average pressure exerted by the molecules on the walls of the container? The Boltzmann constant is 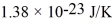 and the ideal gas constant is
and the ideal gas constant is 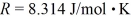 =
= 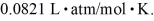
(Multiple Choice)
4.8/5  (30)
(30)
Showing 1 - 20 of 58
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)