Exam 39: Particles Behaving As Waves
Exam 1: Units,physical Quantities,and Vectors107 Questions
Exam 2: Motion Along a Straight Line59 Questions
Exam 3: Motion in Two or Three Dimensions50 Questions
Exam 4: Newtons Laws of Motion44 Questions
Exam 5: Applying Newtons Laws95 Questions
Exam 6: Work and Kinetic Energy54 Questions
Exam 7: Potential Energy and Energy Conservation55 Questions
Exam 8: Momentum,impulse,and Collisions50 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Dynamics of Rotational Motion49 Questions
Exam 11: Equilibrium and Elasticity50 Questions
Exam 12: Fluid Mechanics50 Questions
Exam 13: Gravitation50 Questions
Exam 14: Periodic Motion50 Questions
Exam 15: Mechanical Waves44 Questions
Exam 16: Sound and Hearing65 Questions
Exam 17: Temperature and Heat63 Questions
Exam 18: Thermal Properties of Matter58 Questions
Exam 19: The First Law of Thermodynamics52 Questions
Exam 20: The Second Law of Thermodynamics50 Questions
Exam 21: Electric Charge and Electric Field60 Questions
Exam 22: Gausss Law41 Questions
Exam 23: Electric Potential55 Questions
Exam 24: Capacitance and Dielectrics52 Questions
Exam 25: Current,resistance,and Electromotive Force55 Questions
Exam 26: Direct-Current Circuits53 Questions
Exam 27: Magnetic Field and Magnetic Forces42 Questions
Exam 28: Sources of Magnetic Field52 Questions
Exam 29: Electromagnetic Induction39 Questions
Exam 30: Inductance27 Questions
Exam 31: Alternating Current50 Questions
Exam 32: Electromagnetic Waves47 Questions
Exam 33: The Nature and Propagation of Light28 Questions
Exam 34: Geometric Optics81 Questions
Exam 35: Interference33 Questions
Exam 36: Diffraction49 Questions
Exam 37: Relativity51 Questions
Exam 38: Photons: Light Waves Behaving As Particles38 Questions
Exam 39: Particles Behaving As Waves52 Questions
Exam 40: Quantum Mechanics43 Questions
Exam 41: Atomic Structure53 Questions
Exam 42: Molecules and Condensed Matter31 Questions
Exam 43: Nuclear Physics90 Questions
Exam 44: Particle Physics and Cosmology54 Questions
Select questions type
The energy of the ground state in the Bohr model of the hydrogen atom is -13.6 eV.The energy of the n = 2 state of hydrogen in this model is closest to
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
A
A nonrelativistic electron is accelerated from rest through a potential difference.After acceleration the electron has a de Broglie wavelength of 880 nm.What is the potential difference though which this electron was accelerated? 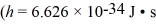 ,
, 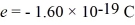 ,
, 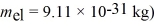
Free
(Multiple Choice)
4.9/5  (43)
(43)
Correct Answer:
A
How fast must a nonrelativistic electron move so its de Broglie wavelength is the same as the wavelength of a 3.4-eV photon? (mel = 9.11 × 10-31 kg,c = 3.00 × 108 m/s, 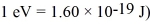
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
A
A molecule of roughly spherical shape has a mass of 6.10 × 10-25 kg and a diameter of 0.70 nm.The uncertainty in the measured position of the molecule is equal to the molecular diameter.What is the minimum uncertainty in the speed of this molecule? 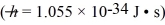
(Multiple Choice)
4.8/5  (32)
(32)
Calculate the kinetic energy (in eV)of a nonrelativistic neutron that has a de Broglie wavelength of 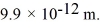 (h = 6.626 × 10-34 J • s,mneutron = 1.675 × 10-27 kg,
(h = 6.626 × 10-34 J • s,mneutron = 1.675 × 10-27 kg, 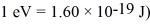
(Short Answer)
4.8/5  (32)
(32)
Light shines through atomic hydrogen gas.It is seen that the gas absorbs light readily at a wavelength of 91.63 nm.What is the value of n of the level to which the hydrogen is being excited by the absorption of light of this wavelength? Assume that the most of the atoms in the gas are in the lowest level.(h = 6.626 × 10-34 J ∙ s,c = 3.00 × 108 m/s, 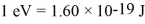 ,the Rydberg constant is R = 1.097 × 107 m-1)
,the Rydberg constant is R = 1.097 × 107 m-1)
(Multiple Choice)
4.8/5  (32)
(32)
A nonrelativistic proton is confined to a length of 2.0 pm on the x-axis.What is the kinetic energy of the proton if its speed is equal to the minimum uncertainty possible in its speed? 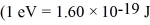 ,h = 1.055 × 10-34 J • s,mproton = 1.67 × 10-27 kg)
,h = 1.055 × 10-34 J • s,mproton = 1.67 × 10-27 kg)
(Multiple Choice)
4.7/5  (32)
(32)
An unstable particle produced in a high-energy collision is measured to have an energy of 483 MeV and an uncertainty in energy of 84 keV.Use the Heisenberg uncertainty principle to estimate the lifetime of this particle.( h = 1.055 × 10-34 J • s = 6.59 × 10-16 eV • s)
(Short Answer)
5.0/5  (31)
(31)
The wavelength of a ruby laser is 694.3 nm.What is the energy difference between the two energy states involved in laser action? (c = 2.9979 × 108 m/s,h = 6.626 × 10-34 J • s, 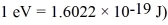
(Multiple Choice)
4.7/5  (42)
(42)
The energy of an electron state has an uncertainty of 0.500 eV.What is the minimum uncertainty in the lifetime of the level? ( h = 1.055 × 10-34 J • s = 6.591 × 10-16 eV • s, 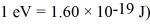
(Multiple Choice)
4.8/5  (34)
(34)
The Bohr radius of the hydrogen atom is 0.529 × 10-10 m.What is the radius of the n = 2 state?
(Multiple Choice)
4.9/5  (42)
(42)
Light excites atomic hydrogen from its lowest level to the n = 4 level.What is the energy of the light? The energy of the lowest level is -13.6 eV.
(Multiple Choice)
4.8/5  (39)
(39)
If the accuracy in measuring the position of a particle increases,the accuracy in measuring its velocity will
(Multiple Choice)
4.8/5  (32)
(32)
A gas of helium atoms (each of mass 6.65 × 10-27 kg)are at room temperature of 20.0°C.What is the de Broglie wavelength of the helium atoms that are moving at the root-mean-square speed? (h = 6.626 × 10-34 J • s,the Boltzmann constant is 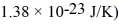
(Multiple Choice)
4.9/5  (36)
(36)
An electric current through a tungsten filament maintains its temperature at 2800 K.Assume the tungsten filament behaves as an ideal radiator at that temperature.If the radiating area of the filament is 2.0 × 10-6 m2,at what rate does it radiate energy? 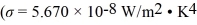 ,Wien displacement law constant is 2.90 × 10-3 m • K)
,Wien displacement law constant is 2.90 × 10-3 m • K)
(Multiple Choice)
4.9/5  (31)
(31)
In a double slit experiment,a beam of electrons strikes a pair of slits.The slits are 15 μm apart,and the first interference maximum lies at an angle of 0.50 µrad from the center of the interference pattern.What is the momentum of the incoming electrons? 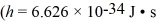 ,mel = 9.11 × 10-31 kg)
,mel = 9.11 × 10-31 kg)
(Multiple Choice)
4.7/5  (32)
(32)
An electron inside a hydrogen atom is confined to within a space of 0.110 nm.What is the minimum uncertainty in the electron's velocity? ( h = 1.055 × 10-34 J • s, 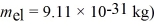
(Multiple Choice)
4.9/5  (32)
(32)
A hydrogen atom makes a downward transition from the 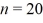 state to the n = 5 state.Find the wavelength of the emitted photon.The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s)
state to the n = 5 state.Find the wavelength of the emitted photon.The lowest level energy state of hydrogen is -13.6 eV. (h = 6.626 × 10-34 J ∙ s,1 eV = 1.60 × 10-19 J,c = 3.00 × 108 m/s)
(Multiple Choice)
4.9/5  (28)
(28)
An electric current through a tungsten filament maintains its temperature at 2800 K.Assume the tungsten filament behaves as an ideal radiator at that temperature.Near what wavelength does the filament emit the greatest power? (σ = 5.67 × 10-8 W/m2 • K4,Wien displacement law constant is 2.9 × 10-3 m • K)
(Multiple Choice)
4.9/5  (40)
(40)
In a ruby laser,an electron jumps from a higher energy level to a lower one.If the energy difference between the two levels is 1.8 eV,what is the wavelength of the emitted photon? 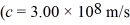 ,h = 6.626 × 10-34 J • s,1 eV = 1.60 × 10-19 J)
,h = 6.626 × 10-34 J • s,1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.9/5  (30)
(30)
Showing 1 - 20 of 52
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)