Exam 8: The Structure of Atoms and Periodic Trends
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
Which of the following orbital diagrams represents a diamagnetic atom?
1s 2s 2p
(Multiple Choice)
5.0/5  (41)
(41)
An atom of which of the following elements has the largest atomic radius?
(Multiple Choice)
5.0/5  (38)
(38)
Which of the following statements concerning ground state electron configurations is/are CORRECT?
1)For a hydrogen atom with one electron,the 2s and 2p orbitals have identical energies.
2)For a lithium atom with three electrons,the 2s and 2p orbitals have different energies.
3)The effective nuclear charge felt by an electron in a 2p orbital is greater for a carbon atom than for a boron atom.
(Multiple Choice)
4.9/5  (34)
(34)
Which element is found in the p-block of the periodic table?
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following cations has the same number of unpaired electrons as Fe2+?
(Multiple Choice)
4.7/5  (37)
(37)
The change in energy for the following reaction is referred to as the ____ for boron.B(g)+ e- B-(g)
(Multiple Choice)
4.8/5  (33)
(33)
The procedure by which electrons are assigned to (or built up into)orbitals is known as the ____ principle.
(Multiple Choice)
4.7/5  (32)
(32)
What 2- ion has the following ground state electron configuration? 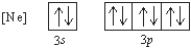
(Multiple Choice)
4.8/5  (47)
(47)
Which of the following atoms is diamagnetic in its ground state?
(Multiple Choice)
4.7/5  (35)
(35)
According to the general trend in electron affinities,which group (or family)of elements tend to form the most stable anions in the gas phase?
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following statements is/are CORRECT?
1)For any element,the second ionization energy is larger than the first ionization energy.
2)Ionization energy is a positive value for all elements.
3)Ionization energy increases down a group of the periodic table.
(Multiple Choice)
4.9/5  (32)
(32)
All of the following ground-state electron configurations are correct except
(Multiple Choice)
4.9/5  (31)
(31)
For which of the following atoms is the 2+ ion diamagnetic in the ground state?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following statements is true concerning the electron configuration [Ar]4p2?
(Multiple Choice)
4.8/5  (34)
(34)
An atom of which of the following elements has the most negative electron affinity?
(Multiple Choice)
4.8/5  (42)
(42)
Which atom has the ground state electronic configuration 1s22s22p63s23p64s23d3?
(Multiple Choice)
4.9/5  (42)
(42)
Rank F,Cl,and Br in order of increasing first ionization energy.
(Multiple Choice)
4.8/5  (42)
(42)
Showing 61 - 80 of 82
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)