Exam 8: The Structure of Atoms and Periodic Trends
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
What is the maximum number of electrons that can occupy the n = 1 shell?
(Multiple Choice)
4.9/5  (37)
(37)
The ground-state electron configuration of a 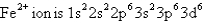 .Therefore,
.Therefore,  Is
Is
(Multiple Choice)
5.0/5  (35)
(35)
Which of the following orbital diagrams represent(s)a paramagnetic atom?
1s 2s 2p
1)  2)
2)  3)
3) 
(Multiple Choice)
4.8/5  (43)
(43)
How many electrons can be described by the following quantum numbers: n = 5, 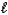 = 0,
= 0,  = 0,ms = -1/2?
= 0,ms = -1/2?
(Multiple Choice)
4.9/5  (33)
(33)
Rank the following ions in order of decreasing ionic radii: Rb-,Br-,Te2-,I-.
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following statements concerning the Pauli exclusion principle is/are CORRECT?
1)If two electrons occupy the same orbital they must have opposite spins.
2)No two electrons in an atom can have the same four quantum numbers.
3)Electrons with opposing spins are attracted to each other.
(Multiple Choice)
4.8/5  (27)
(27)
If the ground state electron configuration of an element is [Ar]3d104s24p5,what is the typical charge on the monatomic anion of the element?
(Multiple Choice)
4.8/5  (30)
(30)
What 2+ ion has the following ground state electron configuration? 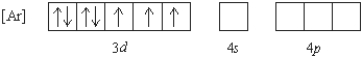
(Multiple Choice)
4.8/5  (40)
(40)
According to the building-up principle or aufbau principle,which subshell is typically filled next after the 5p subshell?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following statements is/are CORRECT for an oxygen atom?
1)The effective nuclear charge felt by a 2s electron is greater than that felt by a 1s electron.
2)The effective nuclear charges felt by 2s and 2p electrons are identical.
3)The effective nuclear charge felt by a 2p electron is less than that felt by a 2s electron.
(Multiple Choice)
4.9/5  (40)
(40)
A metal halide forms when strontium reacts with elemental iodine.What is the most likely formula of this metal halide?
(Multiple Choice)
4.8/5  (47)
(47)
Which element has the ground state electron configuration [Ar]3d104s1?
(Multiple Choice)
4.8/5  (32)
(32)
The element ________ has the following electron configuration: [Rn]5f127s2.
(Short Answer)
4.9/5  (43)
(43)
Which element has the following ground state electron configuration?
3d 4s
[Ar]
![Which element has the following ground state electron configuration? ~~~~~~~~ 3d ~~~~~~~~ ~~~~~~~~ ~~~~~~~~ ~~~~~~~~ ~~~~~~~~ 4s [Ar]](https://storage.examlex.com/TB4499/11ea8937_ab65_7194_a16d_710515c61dbe_TB4499_11.jpg)
(Multiple Choice)
4.9/5  (27)
(27)
What is the ground-state electron configuration of the sulfide ion?
(Multiple Choice)
4.9/5  (37)
(37)
The maximum number of electrons that can be accommodated in an s subshell is
(Multiple Choice)
4.8/5  (37)
(37)
Showing 41 - 60 of 82
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)