Exam 9: Bonding and Molecular Structure
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
The Lewis structure of which of the following formula violates the octet rule?
(Multiple Choice)
4.9/5  (36)
(36)
When you draw the Lewis structure for PF2Cl3,how many single bonds,double bonds,and lone pair electrons reside on the central phosphorus atom?
(Multiple Choice)
4.9/5  (27)
(27)
Which of the following elements is most likely to form a molecular structure that disobeys the octet rule?
(Multiple Choice)
4.9/5  (34)
(34)
Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of boron tribromide,BBr3.
(Multiple Choice)
4.8/5  (39)
(39)
How many lone pairs of electrons are assigned to the iodine atom in ICl?
(Multiple Choice)
4.8/5  (40)
(40)
Use VSEPR theory to predict the molecular geometry around either carbon atom in acetylene,C2H2.
(Multiple Choice)
4.8/5  (35)
(35)
If two or more species have the same number of electrons,resulting in similar Lewis structures,they are said to be ____.
(Multiple Choice)
5.0/5  (43)
(43)
Which combination of atoms is most likely to produce a compound with covalent bonds?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following elements is able to form a molecular structure that exceeds the octet rule?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following has an incomplete octet in its Lewis structure?
(Multiple Choice)
4.7/5  (38)
(38)
How many hydrogen atoms are needed to complete the following hydrocarbon structure? 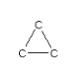
(Multiple Choice)
4.9/5  (34)
(34)
Use the bond energies provided to complete the following statement.________ when all of the bonds in acetic acid (CH3COOH)are formed.
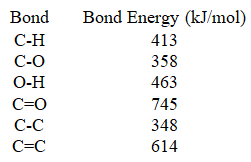
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following species will have a Lewis structure most like that of IF4-?
(Multiple Choice)
4.8/5  (36)
(36)
Which combination of atoms is most likely to produce a compound with ionic bonds?
(Multiple Choice)
4.8/5  (32)
(32)
In which pair do compounds exhibit predominantly ionic bonding?
(Multiple Choice)
4.8/5  (36)
(36)
What is the formal charge on each atom in dichloromethane,CH2Cl2?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following is a correct Lewis structure for sulfate ion?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 21 - 40 of 97
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)