Exam 1: Atomic and Molecular Structure
Exam 1: Atomic and Molecular Structure54 Questions
Exam 2: Interchapter: Nomenclature 1-477 Questions
Exam 3: Interchapter 1molecular Orbital Theory and Chemical Reactions17 Questions
Exam 4: Three-Dimensional Geometry,intermolecular Interactions,and Physical Properties53 Questions
Exam 5: Orbital Interactions 1: Hybridization and Two-Center Molecular Orbitals56 Questions
Exam 6: Isomerism 1: Conformational and Constitutional Isomers60 Questions
Exam 7: Isomerism 2: Chirality,enantiomers,and Diastereomers63 Questions
Exam 8: The Proton Transfer Reaction: an Introduction to Mechanisms,thermodynamics,and Charge Stability51 Questions
Exam 9: An Overview of the Most Common Elementary Steps58 Questions
Exam 10: An Introduction to Multistep Mechanisms: Sn1 and E1 Reactions59 Questions
Exam 11: Nucleophilic Substitution and Elimination Reactions 1: Competition Among Sn2,sn1,e2,and E1 Reactions50 Questions
Exam 12: Nucleophilic Substitution and Elimination Reactions 2: Reactions That Are Useful for Synthesis58 Questions
Exam 13: Electrophilic Addition to Nonpolar Π Bonds 1: Addition of a Brønsted Acid50 Questions
Exam 14: Electrophilic Addition to Nonpolar Π Bonds 2: Reactions Involving Cyclic Transition States55 Questions
Exam 15: Organic Synthesis 1: Beginning Concepts50 Questions
Exam 16: Orbital Interactions 2: Extended Π Systems,conjugation,and Aromaticity54 Questions
Exam 17: Structure Determination 1: Ultravioletvisible and Infrared Spectroscopies50 Questions
Exam 18: Structure Determination 2: Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry60 Questions
Exam 19: Nucleophilic Addition to Polar Π Bonds 1: Addition of Strong Nucleophiles50 Questions
Exam 20: Nucleophilic Addition to Polar Π Bonds 2: Addition of Weak Nucleophiles and Acid and Base Catalysis63 Questions
Exam 21: Organic Synthesis 2: Intermediate Topics in Synthesis Design,and Useful Reduction and Oxidation Reactions50 Questions
Exam 22: Nucleophilic Additionelimination Reactions 1: the General Mechanism Involving Strong Nucleophiles55 Questions
Exam 23: Nucleophilic Additionelimination Reactions 2: Weak Nucleophiles54 Questions
Exam 24: Electrophilic Aromatic Substitution 1: Substitution on Benzene; Useful Accompanying Reactions50 Questions
Exam 25: Electrophilic Aromatic Substitution 2: Substitution on Mono- and Disubstituted Benzene and Other Aromatic Rings50 Questions
Exam 26: The Dielsalder Reaction and Other Pericyclic Reactions53 Questions
Exam 27: Reactions Involving Free Radicals50 Questions
Exam 28: Polymers51 Questions
Select questions type
Using line structures,deduce individual resonance contributors from the resonance hybrid structure given here. 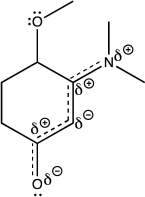
(Essay)
4.9/5  (39)
(39)
Draw all possible resonance forms for anisole using appropriate arrow notation.Which resonance structure is most stable? Least stable? Draw the resonance hybrid for anisole,indicating all partial charges. 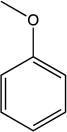
(Essay)
4.9/5  (37)
(37)
Oxygen is an important heteroatom found in many organic molecules.Consider methanol and its protonated derivative,shown below.Indicate relevant bond dipoles using dipole arrows.How does an oxygen with a positive charge,called an oxonium species,influence the magnitude of the partial positive charge on the carbon atom? Which oxygen-carbon bond do you think is more difficult to break? Explain. 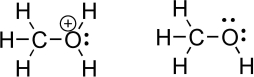
(Essay)
4.7/5  (33)
(33)
Using line structures,draw the individual resonance contributors from the resonance hybrid structure given here. 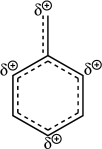
(Essay)
4.8/5  (44)
(44)
Which individual structures below could be contributing resonance structures to the given hybrid structure? 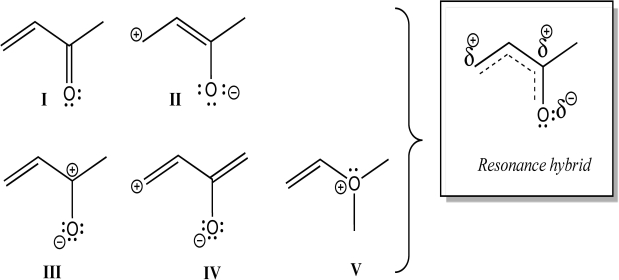
(Multiple Choice)
4.8/5  (38)
(38)
Peptide bonds are the building blocks of proteins.Consider a peptide bond formed from the amino acids alanine and serine,shown below.Draw the resonance forms and the resonance hybrid for the amide bond of the dipeptide.Use appropriate arrow notation. 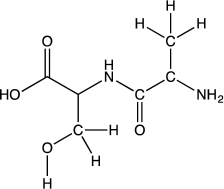
(Short Answer)
4.9/5  (36)
(36)
Which of the following amino acids possesses two hydrogen atoms adjacent to the carboxylic acid?
(Multiple Choice)
4.8/5  (44)
(44)
Which orbital does not house core electrons for a bromine atom?
(Multiple Choice)
4.7/5  (43)
(43)
Identify the molecules below as monosaccharides,disaccharides,or carbohydrates.Explain the distinction between these classes of biologically active organic molecules. 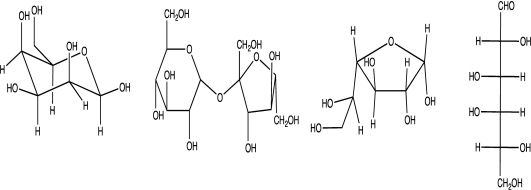
(Essay)
4.8/5  (40)
(40)
Which electron configuration is correct for a carbon atom with a formal charge of -1?
(Multiple Choice)
4.9/5  (26)
(26)
Showing 41 - 54 of 54
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)