Exam 7: Enzyme Mechanisms
Exam 1: Principles of Biochemistry98 Questions
Exam 2: Physical Biochemistry: Energy Conversion,water,and Membranes99 Questions
Exam 3: Nucleic Acid Structure and Function100 Questions
Exam 4: Protein Structure100 Questions
Exam 5: Methods in Protein Biochemistry98 Questions
Exam 6: Protein Function113 Questions
Exam 7: Enzyme Mechanisms105 Questions
Exam 8: Cell Signaling Systems102 Questions
Exam 9: Glycolysis: a Paradigm of Metabolic Regulation100 Questions
Exam 10: The Citrate Cycle100 Questions
Exam 11: Oxidative Phosphorylation98 Questions
Exam 12: Photosynthesis100 Questions
Exam 13: Carbohydrate Structure and Function100 Questions
Exam 14: Carbohydrate Metabolism100 Questions
Exam 15: Lipid Structure and Function98 Questions
Exam 16: Lipid Metabolism100 Questions
Exam 17: Amino Acid Metabolism100 Questions
Exam 18: Nucleotide Metabolism98 Questions
Exam 19: Metabolic Integration101 Questions
Exam 20: Dna Replication, repair, and Recombination99 Questions
Exam 21: Rna Synthesis, processing, and Gene Silencing100 Questions
Exam 22: Protein Synthesis, posttranslational Modification, and Transport100 Questions
Exam 23: Gene Regulation99 Questions
Select questions type
Procathepsin B is a lysosomal protease that is first translated as a proenzyme.On autocleavage it is fully activated.Procathepsin B is
(Multiple Choice)
4.8/5  (39)
(39)
Below is a Lineweaver-Burk plot in which the axis labels have been removed.Interpret the plot to determine the vmax. 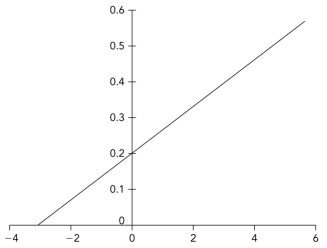
(Multiple Choice)
4.8/5  (43)
(43)
An enzyme that requires the coenzyme nicotinamide adenine dinucleotide belongs to which enzyme class?
(Multiple Choice)
4.9/5  (38)
(38)
Binding of glucose to hexokinase causes a conformational change in the enzyme.This is an example of the __________ model of enzyme catalysis.
(Multiple Choice)
5.0/5  (31)
(31)
The conversion of L-proline to D-proline is shown below.Which of the following characteristics would most likely be found in a transition state analog that inhibits an enzyme that catalyzes the reaction? 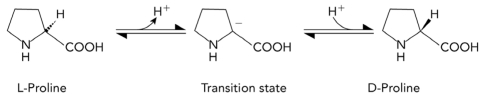
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following is an assumption made when using Michaelis-Menten kinetics?
(Multiple Choice)
4.7/5  (42)
(42)
When compared with the T state of aspartate transcarbamoylase,the R state
(Multiple Choice)
5.0/5  (34)
(34)
Which answer correctly classifies the compound with its relationship to aspartate transcarbamoylase?
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following is true of the tetrahedral intermediate in the chymotrypsin mechanism?
(Multiple Choice)
4.9/5  (36)
(36)
An experiment is performed in which the kinetics of an enzyme-catalyzed reaction at different pHs is monitored.It is found that the Km does not change but that the kcat increases as the pH goes above 7.Which of the following is true?
(Multiple Choice)
4.7/5  (39)
(39)
A plot of vo versus [S] for aspartyl transcarbamoylase displays three sigmoidal lines.If the line in the middle represents the enzyme activity in the absence of any allosteric effectors,then the line to the __________ represents the enzyme in the __________ when bound to __________.
(Multiple Choice)
4.9/5  (33)
(33)
The catalytic triad of chymotrypsin is composed of His57,Ser195,and
(Multiple Choice)
4.8/5  (34)
(34)
The regulation of a biomolecule through the addition or removal of a molecular tag involves __________ reactions.
(Multiple Choice)
4.7/5  (47)
(47)
Propose an experiment to determine the presence of a predicted hydrophobic channel in a newly discovered enzyme.At your disposal you have the purified enzyme,the ability to analyze the enzyme by X-ray crystallography,a spectrophotometer,the enzyme substrate,and polyethylene glycol.Be sure to explain how the results will determine if the protein contains a hydrophobic channel.
(Essay)
4.9/5  (42)
(42)
Connect the use of v0 to the ability to treat k-2 as negligible in Michaelis-Menten kinetics.
(Essay)
4.9/5  (40)
(40)
On a plot of [product] versus time for an enzyme-catalyzed reaction,the v0 is equal to the
(Multiple Choice)
4.9/5  (40)
(40)
Showing 21 - 40 of 105
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)