Exam 7: Enzyme Mechanisms
Exam 1: Principles of Biochemistry98 Questions
Exam 2: Physical Biochemistry: Energy Conversion,water,and Membranes99 Questions
Exam 3: Nucleic Acid Structure and Function100 Questions
Exam 4: Protein Structure100 Questions
Exam 5: Methods in Protein Biochemistry98 Questions
Exam 6: Protein Function113 Questions
Exam 7: Enzyme Mechanisms105 Questions
Exam 8: Cell Signaling Systems102 Questions
Exam 9: Glycolysis: a Paradigm of Metabolic Regulation100 Questions
Exam 10: The Citrate Cycle100 Questions
Exam 11: Oxidative Phosphorylation98 Questions
Exam 12: Photosynthesis100 Questions
Exam 13: Carbohydrate Structure and Function100 Questions
Exam 14: Carbohydrate Metabolism100 Questions
Exam 15: Lipid Structure and Function98 Questions
Exam 16: Lipid Metabolism100 Questions
Exam 17: Amino Acid Metabolism100 Questions
Exam 18: Nucleotide Metabolism98 Questions
Exam 19: Metabolic Integration101 Questions
Exam 20: Dna Replication, repair, and Recombination99 Questions
Exam 21: Rna Synthesis, processing, and Gene Silencing100 Questions
Exam 22: Protein Synthesis, posttranslational Modification, and Transport100 Questions
Exam 23: Gene Regulation99 Questions
Select questions type
Justify how an enzyme that catalyzes a hydrolysis reaction does not contradict the concept that enzyme active sites are microenvironments that exclude excess water.
(Essay)
4.9/5  (30)
(30)
The activity of an enzyme is monitored as a function of temperature.At low temperature very little activity is seen; the activity peaks as the temperature increases and then dramatically decreases to zero as higher temperatures are attained.Explain why the activity drops off completely at higher temperatures.
(Essay)
4.8/5  (39)
(39)
Which of the following is true of the induced-fit model of enzyme catalysis but NOT of the lock and key model of enzyme catalysis?
(Multiple Choice)
4.9/5  (30)
(30)
Which of the following is NOT a primary mechanism that affects catalytic efficiency?
(Multiple Choice)
4.7/5  (40)
(40)
When a nucleophile present in the enzyme attacks an electrophilic substrate to form an enzyme-substrate intermediate,this is an example of __________ catalysis.
(Multiple Choice)
4.9/5  (40)
(40)
Given the following data for lactate dehydrogenase,calculate the specificity constant.
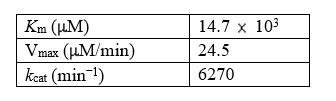
(Essay)
4.9/5  (36)
(36)
The substrate binding pocket of __________ is best at accommodating substrates with small side chains.
(Multiple Choice)
4.7/5  (31)
(31)
Explain the two ways that catalytic efficiency of enzymes can be controlled within a cell.
(Essay)
4.8/5  (41)
(41)
Many medicinal drugs are transition state analogs.They are good drugs because they can interact with the target enzyme active site and are
(Multiple Choice)
4.8/5  (40)
(40)
A reaction coordinate diagram comparing an uncatalyzed reaction with an enzyme-catalyzed reaction can directly illustrate that the enzyme __________,but will not directly illustrate that the enzyme __________.
(Multiple Choice)
4.8/5  (43)
(43)
A plot of 1 / v0 versus 1/[S] is called a __________ plot.Data in this plot have a slope equal to __________.
(Multiple Choice)
4.8/5  (43)
(43)
When __________ is increased,__________ is activated,which acts on glycogen phosphorylase,leading to a decrease in the activity of the enzyme.
(Multiple Choice)
4.8/5  (32)
(32)
An inhibitor that binds only to the ES complex and not free enzyme is known as a(n)__________ inhibitor.
(Multiple Choice)
4.8/5  (32)
(32)
What is the rate enhancement as a result of the presence of an enzyme if the uncatalyzed rate of the reaction is 1.2 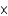 102 mmol/sec and the catalyzed rate is 2.4
102 mmol/sec and the catalyzed rate is 2.4 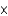 104 mmol/sec?
104 mmol/sec?
(Multiple Choice)
4.8/5  (33)
(33)
The Lineweaver-Burk plot shows data obtained for an enzyme in the absence and presence of a reversible inhibitor.Which type of inhibitor was used in the experiment? 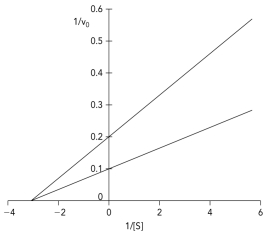
(Multiple Choice)
4.8/5  (41)
(41)
All of the following are common catalytic reaction mechanisms in enzyme active sites EXCEPT __________ catalysis.
(Multiple Choice)
4.9/5  (44)
(44)
Showing 41 - 60 of 105
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)