Exam 21: The Main Group Elements: Life and the Periodic Table
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
The photosynthesis of carbohydrates from carbon dioxide and water is a redox reaction. Metalloenzymes containing manganese assist in the transfer of electrons.
(1) Write the reaction equation for the photosynthetic production of glucose.
(2) Identify the species that is oxidized.
(3) Identify the species that is reduced.
(4) Identify the number of electrons transferred per mole of glucose produced.
Free
(Essay)
4.8/5  (30)
(30)
Correct Answer:
(1) 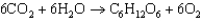
(2) Oxygen goes from 2 to 0, so it is oxidized.
(3) Carbon goes from 4 to 0, so it is reduced.
(4) To produce a molecule of glucose, 6 atoms of carbon must each receive 4 electrons to reduce their oxidation number from 4 to 0. So to produce a mole of glucose, 24 moles of electrons must be transferred.
The average concentration of calcium in the human body is 15 mg/g of body mass. What is the mass of calcium in the body of a person weighing 150 lb? (1 lb 454 g)
Free
(Multiple Choice)
4.7/5  (40)
(40)
Correct Answer:
E
Stomach acid is 0.16 M HCl. What is the pH of stomach acid?
Free
(Multiple Choice)
4.9/5  (41)
(41)
Correct Answer:
A
How much energy must be expended by a cell to transport 1 mole of sodium ions across a membrane with an electrochemical potential of 62 mV in order to maintain this potential?
(Multiple Choice)
4.8/5  (35)
(35)
The cation BiO is found in some over-the-counter antacids. Draw the Lewis structure of this cation that satisfies the octet rule for both atoms. Based on this Lewis structure, this cation has ________ of nonbonding electrons.
(Multiple Choice)
4.8/5  (35)
(35)
The concentration of a trace essential element in the body is typically in the range of ________ per gram of body mass.
(Multiple Choice)
4.9/5  (44)
(44)
Pacemakers implanted in the chests of patients with certain heart conditions are powered by a lithium/iodine PVP battery, where PVP represents polyvinylpyridine. The cell reaction and standard cell potential are given below. The standard reduction potential for Li/Li is 3.05 V. What species is oxidized in this reaction? 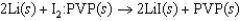 3.59 V
3.59 V
(Multiple Choice)
4.8/5  (33)
(33)
Selenocysteine is an amino acid that gets incorporated into proteins that serve as antioxidants. Evidence points to a need for a minimum daily dose of selenium of 55 g. How many moles of selenocysteine (C3H7NO2Se, 168 g /mol) can be synthesized using this amount of selenium?
(Multiple Choice)
4.8/5  (33)
(33)
Magnesium is found in which one of the following important biological molecules?
(Multiple Choice)
4.9/5  (28)
(28)
Which element, by mass, is most abundant in the human body?
(Multiple Choice)
4.7/5  (33)
(33)
Ions need to move in and out of cells through cell membranes. Direct diffusion of ions through the lipid bilayer of the cell membrane is difficult because ions are not soluble in these nonpolar regions. Describe three mechanisms by which ions are transported through cell membranes.
(Essay)
4.8/5  (31)
(31)
Fluoridation to prevent tooth decay replaces ________ with fluoride in hydroxyapatite.
(Multiple Choice)
4.9/5  (31)
(31)
Plants convert nitrate into ammonia by using enzymes called ________
(Multiple Choice)
4.8/5  (28)
(28)
Hearing aid batteries utilize a zinc/air electrochemical cell. The reduction half-reactions and standard reduction potentials for this cell are given below. What is the standard cell potential of zinc/air cell? 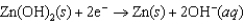 1.249 V
1.249 V 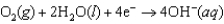
(Short Answer)
4.8/5  (40)
(40)
Which of the following is not a characteristic of a nonessential biological element?
(Multiple Choice)
4.9/5  (26)
(26)
The concentration of sodium in the human body is approximately 1.5 mg/g of body mass. What is this concentration in ppm (parts per million)?
(Multiple Choice)
5.0/5  (33)
(33)
Showing 1 - 20 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)