Exam 1: Matter and Energy: The Origin of the Universe
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Which of the following is the most massive?
Free
(Multiple Choice)
4.8/5  (43)
(43)
Correct Answer:
A
Which one of the following is not a chemical reaction?
Free
(Multiple Choice)
4.7/5  (41)
(41)
Correct Answer:
D
Molecules are represented in various ways. Which statement A-D about molecular representations is not correct.
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following does not represent a physical property of caffeine?
(Multiple Choice)
4.9/5  (42)
(42)
The following measurements of the mass of an aspirin tablet were made by different students in a lab. Which set is the most precise?
(Multiple Choice)
4.8/5  (40)
(40)
In celebration of Mole Day, Turbo the snail competed in a race that was a mole (6.02 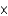 1023) of zeptometers long. If zepto represents a factor of 10-21, how long was the race in meters?
1023) of zeptometers long. If zepto represents a factor of 10-21, how long was the race in meters?
(Multiple Choice)
4.8/5  (32)
(32)
John Dalton postulated that all matter is composed of small particles called atoms. For this proposition to be considered a valid scientific theory, ________
(Multiple Choice)
4.9/5  (30)
(30)
Which one of the following is not a physical process or change?
(Multiple Choice)
4.9/5  (41)
(41)
Spanish mahogany has a density of 53 lb/ft3. Would you be able to lift a piece of mahogany that measured 10 in 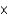 12 in
12 in 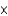 14 in?
14 in?
(Multiple Choice)
4.8/5  (37)
(37)
If an atom is 0.1 nm in diameter, how many atoms must be lined up to make a row 1 cm long?
(Multiple Choice)
4.7/5  (35)
(35)
The density of iron is 7.9 g/cm3. What is the volume of a 4.5 kg iron block?
(Multiple Choice)
4.8/5  (31)
(31)
Which statement A-D about the reaction of methane with oxygen, which is called combustion and is represented by the reaction equation below, is not correct? The reaction products are carbon dioxide and water. CH4 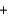 2O2
2O2  CO2
CO2 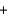 2H2O
2H2O
(Multiple Choice)
4.7/5  (29)
(29)
Showing 1 - 20 of 103
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)