Exam 21: The Main Group Elements: Life and the Periodic Table
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Enzymes in plants convert nitrate into ammonia. The unbalanced half-reaction equation is given below. What is the stoichiometric coefficient of the electrons, e, in the balanced reaction equation? 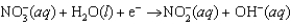
(Multiple Choice)
4.9/5  (44)
(44)
Superoxide dismutase is an enzyme that converts the reactive superoxide ion to hydrogen peroxide. Based on the equation for the reaction shown below, identify the reducing and oxidizing agents in the reaction. 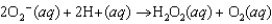
(Essay)
4.7/5  (40)
(40)
The isotope 201Th is used in the diagnosis of heart disease. It has a first-order rate constant, k, of 9.49 103 h1. What is the half-life for 201Th?
(Multiple Choice)
4.9/5  (39)
(39)
Hydroxyapatite, which is a component of tooth enamel, is very insoluble in water. For the following reaction, Ksp 2.3 1059. 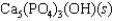
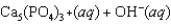 What is the molar concentration of Ca5(PO4)3 when the pH of the mouth is 4.0?
What is the molar concentration of Ca5(PO4)3 when the pH of the mouth is 4.0?
(Multiple Choice)
4.8/5  (40)
(40)
Plants convert nitrogen into ammonia. This biological nitrogen fixation is effected by enzymes called nitrogenases. The enzymes assist in the transfer of electrons. The unbalanced reaction equation is 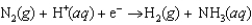 What is the stoichiometric coefficient for ammonia in the balanced reaction equation?
What is the stoichiometric coefficient for ammonia in the balanced reaction equation?
(Multiple Choice)
4.9/5  (36)
(36)
A radioactive isotope that emits the shortest range particles and therefore would be a good choice for implantation therapy most likely decays via ________
(Multiple Choice)
5.0/5  (37)
(37)
Which metal, by the number of atoms, is most abundant in the human body?
(Multiple Choice)
4.7/5  (39)
(39)
Thallium-201 is used in cardiac imaging. It decays by electron capture. What isotope is produced when thallium-201 captures an electron?
(Short Answer)
4.9/5  (29)
(29)
Ion channels in cell membranes control selective transport of ions based on what property(ies)?
(Multiple Choice)
4.8/5  (43)
(43)
Which statement describing the mechanisms by which sodium ions pass through cell membranes is not correct?
(Multiple Choice)
4.9/5  (26)
(26)
Nitrogen is a major essential element in the human body. Which biomolecules incorporate nitrogen?
(Short Answer)
4.9/5  (34)
(34)
Adding fluoride ions to toothpaste and drinking water converts the mineral in tooth enamel, hydroxyapatite, to fluoroapatite. This conversion is beneficial in preventing tooth decay because fluoroapatite ________
(Multiple Choice)
4.9/5  (39)
(39)
Calcium carbonate is used to form exoskeletons. It has a solubility-product constant of 5.0 10 9. What is the molar concentration of calcium ions in a saturated solution of calcium carbonate?
(Multiple Choice)
4.9/5  (40)
(40)
Hydroxyapatite, which is a component of tooth enamel, is very insoluble in water. For the following reaction, Ksp 2.3 1059. 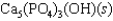
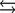
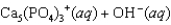 What is the molar concentration of Ca5(PO4)3 when the pH of the mouth is 4.0?
What is the molar concentration of Ca5(PO4)3 when the pH of the mouth is 4.0?
(Short Answer)
4.9/5  (32)
(32)
Different concentrations of ions on either side of a cell membrane constitute a concentration cell, which is described by the Nernst equation given below. A cell must expend energy to maintain this concentration gradient by moving ions from one side of the membrane to the other. How much energy must be expended to transport 1 mole of sodium ions across a membrane when the sodium ion concentration is 0.10 M on one side of the membrane and 0.010 M on the other side, and the temperature is 37C (body temperature)?
(Given: 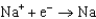 ,
,  2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K))
E E -
2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K))
E E - 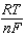 InQ
InQ
(Short Answer)
4.8/5  (34)
(34)
Which of the following elements, by mass, is the most abundant in the universe?
(Multiple Choice)
4.8/5  (41)
(41)
The nucleus of a cell has a pH of approximately 7.3. What is the molar concentration of hydroxide in the nucleus of a cell?
(Multiple Choice)
4.8/5  (42)
(42)
Radioactive tritium, 3H, is used as a tracer in many biological experiments. It decays by emission. What is the product of this decay?
(Multiple Choice)
4.9/5  (36)
(36)
Showing 61 - 80 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)