Exam 21: The Main Group Elements: Life and the Periodic Table
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Selenium can substitute for sulfur in the amino acid cysteine to make the amino acid selenocysteine because ________
(Multiple Choice)
4.9/5  (30)
(30)
Different concentrations of ions on either side of a cell membrane constitute a concentration cell, which is described by the Nernst equation given below. What is the electrochemical potential across a membrane when the sodium ion concentration is 0.10 M on one side of the membrane and 0.010 M on the other side, and the temperature is 37C (body temperature)?
(Given: Na e a, 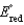 2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (molK))
E E -
2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (molK))
E E - 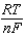 InQ
InQ
(Short Answer)
4.9/5  (38)
(38)
Which element may contribute to sudden infant death syndrome (SIDS), as it is used as a fire retardant in mattresses?
(Multiple Choice)
4.9/5  (42)
(42)
Radioactive 90Sr can substitute for ________ in the human body.
(Multiple Choice)
5.0/5  (35)
(35)
Fluoride is added to toothpastes and drinking water to inhibit tooth decay. What does fluoride do that makes it effective in inhibiting tooth decay?
(Essay)
4.8/5  (40)
(40)
Why are some elements in the human body considered to be nonessential?
(Essay)
4.9/5  (35)
(35)
Pacemakers implanted in the chests of patients with certain heart conditions are powered by a lithium/iodine PVP battery, where PVP represents polyvinylpyridine. The cell reaction and standard cell potential are given below. The standard reduction potential for Li/Li is 3.05 V. What is the standard reduction potential for the iodine PVP half-reaction? 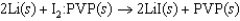 3.59 V
3.59 V
(Multiple Choice)
4.8/5  (37)
(37)
Tooth enamel is composed of the mineral hydroxyapatite. It is essentially insoluble in water with Ksp 2.3 1059, but it reacts with weak acids in the mouth as described by one of the reaction equations below. You can determine the equilibrium constant, K, for this reaction from Ksp and Kw (the equilibrium constant for water autoionization). How do you do this?
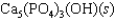
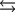
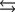
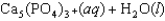
(Multiple Choice)
4.8/5  (34)
(34)
The concentration of an ultratrace essential element in the body is less than ________ per gram of body mass.
(Multiple Choice)
4.8/5  (33)
(33)
Hearing aid batteries utilize a zinc/air electrochemical cell. The reduction half-reactions and standard reduction potentials for this cell are given below. Write the balanced reaction equation for the cell and report the sum of the stoichiometric coefficients. 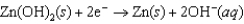 1.249 V
1.249 V 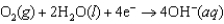 0.401 V
0.401 V
(Multiple Choice)
4.8/5  (34)
(34)
Enzymes in plants convert nitrate into ammonia. The unbalanced half-reaction equation is given below. What is the stoichiometric coefficient of the nitrite ion in the balanced reaction equation? 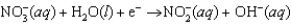
(Multiple Choice)
4.8/5  (38)
(38)
Plants convert nitrogen into ammonia. This biological nitrogen fixation is effected by enzymes called nitrogenases. The enzymes assist in the transfer of electrons. Balance this reaction equation. 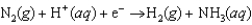
(Essay)
4.8/5  (39)
(39)
Draw two possible Lewis structures of the cation BiO, which is found in some over-the-counter antacids. Which one has the formal charge of 1 on the more electropositive atom?
(Essay)
4.7/5  (39)
(39)
Hearing aid batteries utilize a zinc/air electrochemical cell. The reduction half-reactions and standard reduction potentials for this cell are given below. What species are oxidized and reduced in the cell reaction? 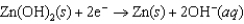 1.249 V
1.249 V 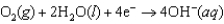 0.401 V
0.401 V
(Multiple Choice)
4.8/5  (28)
(28)
The isotope 18F is used in a range of radio imaging applications. It has a first-order rate constant, k, of 6.30 103 min1. What is the half-life for 18F?
(Multiple Choice)
4.8/5  (40)
(40)
Why are the radioactive isotopes of cesium and strontium that are produced in nuclear reactors or by the atmospheric testing of nuclear weapons especially dangerous to humans?
(Essay)
4.7/5  (33)
(33)
Different concentrations of ions on either side of a cell membrane constitute a concentration cell, which is described by the Nernst equation given below. What is the electrochemical potential across a membrane when the sodium ion concentration is 0.10 M on one side of the membrane and 0.010 M on the other side, and the temperature is 37C (body temperature)? (Given: 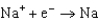 ,
, 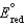 2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K))
E E -
2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K))
E E - 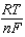 InQ
InQ
(Multiple Choice)
4.8/5  (37)
(37)
The concentration of a major essential element in the body is greater than ________ per gram of body mass.
(Multiple Choice)
4.9/5  (43)
(43)
A patient is injected with a 5.0 M solution of gallium citrate containing radioactive gallium-68 for a PET study. The half-life of gallium-68 is 9.4 hr. How long does it take for the activity of this isotope to drop to 1% of its initial value?
(Multiple Choice)
4.8/5  (42)
(42)
Showing 41 - 60 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)