Exam 5: Thermochemistry: Energy Changes in Reactions
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
The following diagrams illustrate the flow of energy (q) and work (w) in different processes. Which one is definitely an exothermic process?
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
C
Thermochemistry is the study of how ________ is produced and consumed during chemical reactions.
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
C
Ethanol (CH3CH2OH) has been suggested as an alternative fuel source. Ethanol's enthalpy of combustion is Hcomb 1,368 kJ/mol, and its density is 0.789 g/mL. What is the fuel density of ethanol (kJ/mL)?
Free
(Multiple Choice)
5.0/5  (34)
(34)
Correct Answer:
D
Which of the following hydrocarbons has the lowest fuel value?
(Multiple Choice)
4.7/5  (35)
(35)
Sports trainers use cold packs containing ammonium nitrate for injured athletes. Calculate the change in temperature when 47 g of ammonium nitrate (NH4NO3, 80.1 g/mol) dissolves in 100 g water. Assume the specific heat of the solution is 4.5 J/(g C).
NH4NO3(s)  NH4(aq)
NH4(aq) 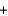 NO3(aq) H 21.1 kJ/mol
NO3(aq) H 21.1 kJ/mol
(Short Answer)
4.8/5  (34)
(34)
Which of the following fuels has the lowest fuel value (kJ/g)?
(Multiple Choice)
4.8/5  (28)
(28)
Given the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. 2N2H4(g) 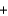 2NO2(g)
2NO2(g)  3N2(g)
3N2(g) 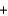 4H2O(g) Hrxn ? kJ
SubstanceH
4H2O(g) Hrxn ? kJ
SubstanceH 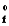 in kJ/mol
N2H4(g)95.4
NO2(g)33.1
H2O(g)241.8
in kJ/mol
N2H4(g)95.4
NO2(g)33.1
H2O(g)241.8
(Multiple Choice)
5.0/5  (35)
(35)
What is the kinetic energy of a skier weighing 175 lb traveling 60 mph? (2.205 lb = 1 kg, 1 mi = 1.609 km, 1 J = 1 kg m2s-2)
(Multiple Choice)
4.8/5  (33)
(33)
Which of the changes A-D will always increase the internal energy of a system?
(Multiple Choice)
4.8/5  (41)
(41)
If a chemical reaction causes the temperature of the container to drop, it is a(n) ________ reaction.
(Multiple Choice)
4.8/5  (34)
(34)
An expanding gas does 175 kJ of work on its surroundings at a constant pressure of 5.55 atm. If the gas initially occupied 125 mL, what is the final volume of the gas? (101.3 J 1 L atm)
(Multiple Choice)
4.9/5  (34)
(34)
When solid sodium hydroxide (NaOH) pellets are dissolved in water, the temperature of the water and beaker rises. This is an example of ________
(Multiple Choice)
4.8/5  (49)
(49)
The complete combustion of 2.500 g of cinnamaldehyde (C9H8O, 132.16 g/mol), which is a component in cinnamon, produces an increase in temperature of 26.65C in a bomb calorimeter (Ccal 3.640 kJ/C). What is the molar enthalpy of combustion of cinnamaldehyde in kJ/mol?
(Short Answer)
4.9/5  (38)
(38)
The heating curve for a substance is shown below. The substance initially is a solid. It then becomes a liquid and a gas. Which of the line segments (I-V) represents the solid-to-liquid phase transition? 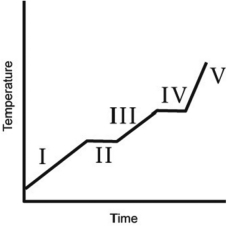
(Multiple Choice)
4.9/5  (40)
(40)
A food sample was burned in a bomb calorimeter containing 524 mL water. How much thermal energy was produced when the temperature of the water and the calorimeter rose from 20.0C to 25.0C? The metal calorimeter had a heat capacity of 725 J/C without the water. The specific heat capacity of water is 4.184 J/(g C).
(Multiple Choice)
4.8/5  (43)
(43)
What is the change in internal energy (E) when a system is heated with 35 J of energy while it does 15 J of work?
(Multiple Choice)
4.7/5  (35)
(35)
Determine the enthalpy for the following reaction, given 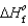 (NH3(g)) 46.1 kJ/mol,
(NH3(g)) 46.1 kJ/mol, 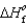 (NO(g))
(NO(g)) 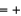 90.3 kJ/mol, and
90.3 kJ/mol, and 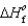 (H2O(g))
(H2O(g))  241.8 kJ/mol.
4NH3(g)
241.8 kJ/mol.
4NH3(g)  5O2(g)
5O2(g)  4NO(g)
4NO(g) 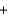 6H2O(g) Hrxn
6H2O(g) Hrxn  ? kJ
? kJ
(Short Answer)
4.9/5  (40)
(40)
Showing 1 - 20 of 139
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)