Exam 21: The Main Group Elements: Life and the Periodic Table
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Which element, by the number of atoms, is least abundant in the human body?
(Multiple Choice)
4.7/5  (36)
(36)
Calcium is a major essential element in the human body. What is the dominant role played by calcium?
(Short Answer)
4.9/5  (31)
(31)
Copper is a trace metal in the human body with a typical concentration of 110 mg in 75 kg of body mass. What is this concentration in units of parts per million (ppm)?
(Multiple Choice)
4.8/5  (40)
(40)
Vanadium is an ultratrace metal in the human body with a typical concentration of approximately 17 ng/g body mass. How many milligrams of V are there in a person weighing 185 lb? 1 lb 454 g
(Multiple Choice)
4.8/5  (39)
(39)
Urea is a waste product produced when the body metabolizes protein. What is the oxidation number of nitrogen in urea, (NH2)2CO?
(Multiple Choice)
4.8/5  (32)
(32)
Enzymes in plants convert nitrate into ammonia. The unbalanced half-reaction equation is given below. What is the stoichiometric coefficient of water in the balanced reaction equation? 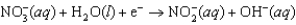
(Multiple Choice)
4.8/5  (39)
(39)
Plants react urea with water to produce ammonia and carbon dioxide. This reaction is an example of a ________ reaction.
(Multiple Choice)
4.8/5  (39)
(39)
A patient is injected with a 5.0 M solution of gallium citrate containing radioactive gallium-68 for a PET study. The half-life of gallium-68 is 9.4 hr. How long does it take for the activity of this isotope to drop to 1% of its initial value?
(Multiple Choice)
4.8/5  (45)
(45)
Enzymes in plants convert nitrate into ammonia. The unbalanced half-reaction equation is given below. What is the change in oxidation number of nitrogen in this reaction? 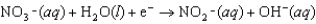
(Multiple Choice)
4.9/5  (38)
(38)
Rhenium-186 has medical applications. It decays by emission. What isotope is formed from this decay?
(Multiple Choice)
4.8/5  (36)
(36)
Selenium is an ultratrace element that is essential for life. It is used in the synthesis of ________, which is ________.
(Multiple Choice)
4.8/5  (37)
(37)
Hearing aid batteries utilize a zinc/air electrochemical cell. The reduction half-reactions and standard reduction potentials for this cell are given below. What is the standard cell potential of a zinc/air cell? 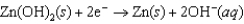 1.249 V
1.249 V 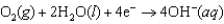 0.401 V
0.401 V
(Multiple Choice)
4.8/5  (39)
(39)
Tooth enamel is composed of the calcium compound called ________
(Multiple Choice)
4.8/5  (32)
(32)
Which metal, by either mass or number of atoms, is most abundant in the human body?
(Multiple Choice)
4.8/5  (39)
(39)
Different concentrations of ions on either side of a cell membrane constitute a concentration cell, which is described by the Nernst equation given below. A cell must expend energy to maintain this concentration gradient by moving ions from one side of the membrane to the other. How much energy must be expended to transport 1 mole of sodium ions across a membrane when the sodium ion concentration is 0.10 M on one side of the membrane and 0.010 M on the other side, and the temperature is 37C (body temperature)? (Given: 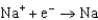 ,
, 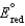 2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K))
E E -
2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K))
E E - 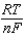 InQ
InQ
(Multiple Choice)
4.8/5  (36)
(36)
Showing 81 - 96 of 96
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)