Exam 10: Intermolecular Forces: The Uniqueness of Water
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Which of the following statements does not correctly identify a factor that affects the boiling point of a pure substance?
I. vapor pressure of the liquid
II. strength of intermolecular forces
III. enthalpy of vaporization
IV. surface area of the liquid
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
D
Portable lanterns and stoves used for camping often use a mixture of hydrocarbons for fuel. One such hydrocarbon is an alkane called n-pentane. These lanterns and stoves are often difficult to light on a cold day because the fuel has a low vapor pressure at low temperatures. Determine the vapor pressure of n-pentane on a night when the temperature is 0.0C. The enthalpy of vaporization of n-pentane is 27.6 kJ/mol, and its boiling point is 36.0C.
Free
(Multiple Choice)
4.8/5  (45)
(45)
Correct Answer:
C
Which alkane compound has the highest vapor pressure?
Free
(Multiple Choice)
4.7/5  (33)
(33)
Correct Answer:
A
The phase diagram for carbon dioxide is shown below. What is the phase that exists at room temperature and pressure (22oC, 1 atm)? 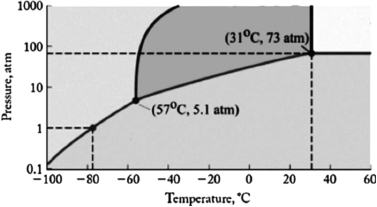
(Multiple Choice)
4.9/5  (47)
(47)
Which one of the following substances would you predict to have the highest vapor pressure at a given temperature? In these line drawings, a carbon is implicit at the end of a line (bond) or where two or more lines come together. Carbon requires four bonds, so any missing bonds are implicitly bonds to hydrogen atoms.
(Multiple Choice)
4.8/5  (45)
(45)
Which of the following substances would you expect to have the largest van der Waals a constant value?
(Multiple Choice)
4.8/5  (37)
(37)
Which one of the following substances would you predict to have the highest boiling point? In these line drawings, a carbon is implicit at the end of a line (bond) or where two or more lines come together. Carbon requires four bonds, so any missing bonds are implicitly bonds to hydrogen atoms.
(Multiple Choice)
5.0/5  (40)
(40)
The relative energies (strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below. Which substance has the highest melting point? 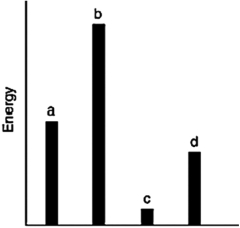
(Multiple Choice)
4.8/5  (30)
(30)
Would water rise to the same height in a capillary tube made of polyethylene plastic as it does in a glass capillary tube of the same diameter? Polyethylene is a hydrocarbon, and glass is mostly silicon dioxide. Explain.
(Essay)
4.8/5  (41)
(41)
Gasoline is primarily a mixture of hydrocarbons and is sold with an octane rating that is based on a comparison with the combustion properties of isooctane. Gasoline usually contains an isomer of isooctane called tetramethylbutane (C8H18), which has an enthalpy of vaporization of 43.3 kJ/mol and a boiling point of 106.5C. Determine the vapor pressure of tetramethylbutane on a very hot summer day when the temperature is 38C.
(Multiple Choice)
4.8/5  (38)
(38)
The temperature at point a in the phase diagram below is the ________ 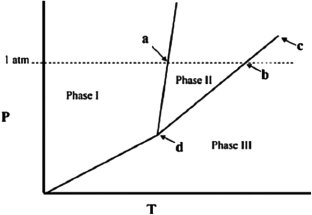
(Multiple Choice)
4.8/5  (44)
(44)
The pressure of carbon dioxide in a beer bottle is about 2 atm. At this pressure about 0.15 g of carbon dioxide dissolves in 100 mL of beer. How much carbon dioxide remains dissolved in 100 mL after the bottle is opened? The partial pressure of carbon dioxide is 2.0 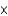 104 atm.
104 atm.
(Multiple Choice)
4.8/5  (28)
(28)
Which liquid, water or ethanol, would you expect to have the higher surface tension and viscosity? Explain.
(Essay)
4.9/5  (33)
(33)
Water forms a concave meniscus in a glass tube because ________
(Multiple Choice)
4.8/5  (33)
(33)
The relative energies (strengths) of the intermolecular forces present in each of four different pure gases are shown in the figure below.
a) Which gas will show the smallest deviation from ideal gas behavior?
b) Which substance has the lowest melting point?
c) Which substance is most likely to be a solid at room temperature? 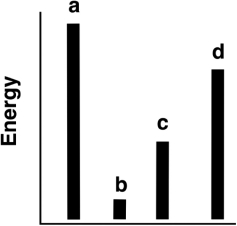
(Essay)
4.9/5  (38)
(38)
Showing 1 - 20 of 165
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)