Exam 9: Molecular Geometry: Shape Determines Function
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
What is the valence electron molecular orbital electron configuration of O2?
(Multiple Choice)
4.9/5  (32)
(32)
Identify the hybridization of the atomic orbitals on carbon atoms labeled 1, 2, and 3. The hydrogen atoms and lone-pair electrons are not shown in the diagram. 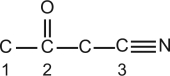
(Essay)
4.8/5  (39)
(39)
Which one of the following molecules is paramagnetic? These molecules are described by the MO energy diagram below. These molecular orbitals are formed from the 2s and 2p atomic orbitals. 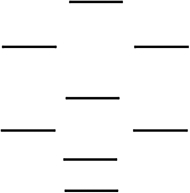
(Multiple Choice)
4.9/5  (40)
(40)
A triatomic molecule with a bond angle close to 120o could be ________
(Multiple Choice)
4.9/5  (37)
(37)
Identify the molecular geometry of the molecular anion, SF5-, and the hybridization of the S atomic orbitals.
(Short Answer)
4.9/5  (46)
(46)
For the series methane, ammonia, and water, the bond angle increases in the following order: H2O NH3 CH4. This trend is due to ________
(Multiple Choice)
4.7/5  (42)
(42)
Which of the following molecules has a central atom that is sp3 hybridized?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following compounds has a square pyramid geometry?
(Multiple Choice)
4.8/5  (39)
(39)
Identify the molecular geometry of the nitrate ion, NO3, and the hybridization of the N atomic orbitals.
(Short Answer)
4.7/5  (31)
(31)
What is the molecular geometry around a central atom having three single bonds and two lone pairs (i.e., nonbonding pairs)?
(Multiple Choice)
4.7/5  (37)
(37)
What is the molecular geometry around a central atom having four single bonds and one lone pair (i.e., nonbonding pair)?
(Multiple Choice)
4.9/5  (33)
(33)
In VSEPR theory, molecular geometry is determined by ________
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following shows the orientation of the net dipole for OCS?
(Multiple Choice)
4.8/5  (44)
(44)
Boron nitride, BN, is a new high-tech material. According to the molecular orbital energy-level diagram below, which one of the following statements is not correct about boron nitride? These molecular orbitals are formed from the 2s and 2p atomic orbitals. 
(Multiple Choice)
4.9/5  (33)
(33)
Which type of molecular orbital contains a node perpendicular to the axis connecting two atomic nuclei?
(Multiple Choice)
5.0/5  (31)
(31)
Showing 101 - 120 of 188
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)