Exam 1: Matter and Energy: The Origin of the Universe
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Indicate which of the following common laboratory devices will deliver 25 mL of a solution with the greatest precision.
(Multiple Choice)
4.8/5  (37)
(37)
Aroldis Chapman is an MLB relief pitcher who recorded the fastest pitch in a major league game, which was clocked at 105 mi per hour. What was this speed in m/s? (1 mi  1.609 km)
1.609 km)
(Multiple Choice)
4.8/5  (40)
(40)
Which one of the following is not equal to exactly one cubic meter (1 m3)?
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following quantities has two significant figures?
(Multiple Choice)
4.7/5  (42)
(42)
Table sugar (sucrose) with the formula C12H22O11 is ________
I. an element.
II. a compound.
III. a mixture.
(Multiple Choice)
4.9/5  (39)
(39)
To bake a cake, it requires 16 teaspoons of vegetable oil. How many fluid ounces is that? (1 cup  48 teaspoons and 1 cup
48 teaspoons and 1 cup  8 fl oz)
8 fl oz)
(Multiple Choice)
4.7/5  (36)
(36)
Cheetahs can run at speeds of up to 60 mi per hour. How many seconds does it take a cheetah to run 10 m at this speed? (1 mi  1.609 km)
1.609 km)
(Multiple Choice)
4.8/5  (38)
(38)
During winter in Siberia, a tenant in a high-rise apartment building dumped a liter of steam (gaseous water) from a container off his balcony. Before it reached the ground, solid snow formed without observation of liquid water. The phase transition described by this process is called ________
(Multiple Choice)
4.7/5  (38)
(38)
What change of state is represented by the following diagram? 
(Multiple Choice)
4.7/5  (42)
(42)
On a summer day, the temperature in Phoenix, Arizona, was recorded as 110  F. What is this temperature in
F. What is this temperature in  C?
C?
(Multiple Choice)
4.9/5  (35)
(35)
Electromagnetic radiation in the mid-infrared region of the spectrum has wavelengths around 0.6 m. Express this wavelength in meters using exponential notation (1 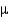 m
m  10-6 m).
10-6 m).
(Multiple Choice)
4.8/5  (43)
(43)
The measurement 54.40 m contains ________ significant figure(s).
(Multiple Choice)
4.9/5  (35)
(35)
A burette (shown below) was used to add dilute hydrochloric acid (HCl) to a solution containing sodium hydroxide (NaOH). If the burette initially was read as 0.00 mL, how much HCl has been delivered according to the reading in the figure? 
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following statements correctly describes the properties of a liquid?
(Multiple Choice)
4.9/5  (38)
(38)
Which statement A-D about accuracy and precision is not correct?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 41 - 60 of 103
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)