Exam 1: Matter and Energy: The Origin of the Universe
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Which of the following is not a chemical property of chlorine?
(Multiple Choice)
4.9/5  (48)
(48)
In 1 second, light can travel 2.998 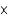 108 m. How many inches does light travel in 1 femtosecond? (1 fs
108 m. How many inches does light travel in 1 femtosecond? (1 fs  10-15 s, 1 inch
10-15 s, 1 inch  2.54 cm exactly)
2.54 cm exactly)
(Multiple Choice)
4.9/5  (33)
(33)
Which diagram depicts the process of sublimation (an asterisk denotes the initial phase of the substance)?
(Multiple Choice)
4.8/5  (39)
(39)
A significant difference in boiling points of two liquids could provide the basis for separation by what physical method?
(Multiple Choice)
4.8/5  (44)
(44)
The summit of Mt. Humphreys, the highest point in Arizona, is 12,600 ft. How many meters is this? (1 m  1.0936 yd, 1 yd
1.0936 yd, 1 yd  3 ft exactly)
3 ft exactly)
(Multiple Choice)
4.8/5  (41)
(41)
What would you report for the total mass of three samples weighing 106.2 g, 33.15 g, and 0.028 g?
(Multiple Choice)
5.0/5  (33)
(33)
What change of state is represented by the following diagram? 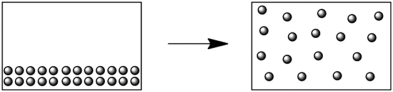
(Multiple Choice)
4.9/5  (29)
(29)
The symbol and name corresponding to the factor 10-6 is ________
(Multiple Choice)
4.7/5  (38)
(38)
A temperature of 78  F was recorded yesterday. The forecasted high for today is predicted to be above this temperature. Which of the following could be the expected high temperature?
F was recorded yesterday. The forecasted high for today is predicted to be above this temperature. Which of the following could be the expected high temperature?
(Multiple Choice)
4.9/5  (39)
(39)
What is the temperature in  C of a reaction mixture that is at 234 K?
C of a reaction mixture that is at 234 K?
(Multiple Choice)
4.8/5  (38)
(38)
An example of a chemical property of formaldehyde (CH2O) is ________
(Multiple Choice)
4.9/5  (42)
(42)
Showing 21 - 40 of 103
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)