Exam 1: Matter and Energy: The Origin of the Universe
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Three metals, and their densities, are shown below. If each were added to the same volume of water (density  1.00 cm3), which metal(s) would sink?
1.00 cm3), which metal(s) would sink? 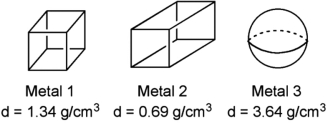
(Multiple Choice)
4.8/5  (33)
(33)
At what temperature do the Celsius and Fahrenheit scales read the same?
(Multiple Choice)
4.8/5  (36)
(36)
Jupiter's mass is estimated to be 1.90 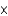 1027 kg, and it has a diameter of 142,984 km. Assuming that Jupiter is spherical, estimate its density (the volume of a sphere is (4
1027 kg, and it has a diameter of 142,984 km. Assuming that Jupiter is spherical, estimate its density (the volume of a sphere is (4 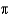 r3) /3).
r3) /3).
(Multiple Choice)
4.7/5  (28)
(28)
Filtration can be used to separate components in a mixture based on differences in ________
(Multiple Choice)
4.8/5  (35)
(35)
If the following operations are carried out, how many significant figures should be reported in the answer? 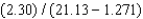
(Multiple Choice)
4.8/5  (39)
(39)
Which technique would allow separation of the following substances? 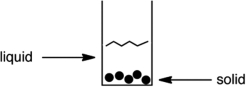
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following mixtures can be separated by filtration?
(Multiple Choice)
4.7/5  (39)
(39)
An irregularly shaped metal object with a mass of 25.43 g was placed in a graduated cylinder with water. The before and after volumes are shown below. What is the density of the metal? 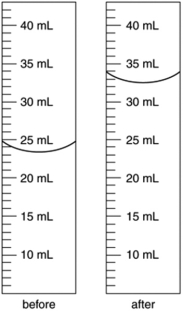
(Multiple Choice)
4.9/5  (34)
(34)
Which diagram shown below represents condensation (an asterisk denotes the initial phase of the substance)?
(Multiple Choice)
5.0/5  (40)
(40)
If you had equal masses of each of the following substances, which would occupy the greatest volume?
(Multiple Choice)
4.7/5  (37)
(37)
Which statement A-D about the reaction of nitrogen monoxide with oxygen, which is called combustion and is represented below by the following cartoon, is not correct? The reaction product is nitrogen dioxide. 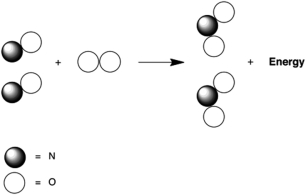
(Multiple Choice)
4.9/5  (34)
(34)
When you place a piece of dry ice (solid carbon dioxide) on a plate at room temperature, you notice that no liquid forms, unlike ice that melts to form liquid water. This is because dry ice ________
(Multiple Choice)
4.9/5  (47)
(47)
Deposition is the process in which a ________ is converted into a ________.
(Multiple Choice)
4.9/5  (40)
(40)
Room temperature is often taken to be 25  C. What is this temperature in
C. What is this temperature in  F?
F?
(Multiple Choice)
4.7/5  (38)
(38)
Identify the incorrect statement(s). A solution ________
I. can be a solid, liquid, or gas.
II. can be heterogeneous or homogeneous.
III. is a homogeneous mixture.
(Multiple Choice)
4.8/5  (31)
(31)
Medicines usually are dispensed in units of mg. What is the mass of a 50 mg tablet?
(Multiple Choice)
4.9/5  (39)
(39)
Showing 61 - 80 of 103
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)