Exam 13: Structure Determination
Exam 1: Structure and Bonding:acids and Bases40 Questions
Exam 2: Alkanes: The Nature of Organic Compounds47 Questions
Exam 3: Alkenes and Alkynes: the Nature of Organic Reactions37 Questions
Exam 4: Reactions of Alkenes and Alkynes44 Questions
Exam 5: Aromatic Compounds51 Questions
Exam 6: Sterechemistry at Tetrahedral Centers35 Questions
Exam 7: Organohalides: Nucleophilic Substitutions and Eliminations49 Questions
Exam 8: Alcohols, Phenols, Ethers, and Their Sulfur Analogs39 Questions
Exam 9: Aldehydes and Ketones: Nucleophilic Addition Reactions32 Questions
Exam 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions66 Questions
Exam 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions37 Questions
Exam 12: Amines31 Questions
Exam 13: Structure Determination64 Questions
Exam 14: Biomolecules: Carbohydrates41 Questions
Exam 15: Biomolecules: Amino Acids, Peptides, and Proteins43 Questions
Exam 16: Biomolecules: Lipids and Nucleic Acids37 Questions
Exam 17: The Organic Chemistry of Metabolic Pathways35 Questions
Select questions type
Which of the following compounds would show the longest wavelength lmax in its UV spectrum? a.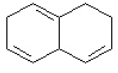 b.
b.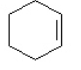 c.
c.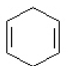 d.
d.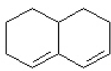
(Short Answer)
4.8/5  (39)
(39)
What are the units for electromagnetic radiation used in infrared spectroscopy?
(Multiple Choice)
4.9/5  (45)
(45)
Which type of spectroscopy (IR, UV, or MS) will best distinguish between the pair of compounds below? Give a brief reason. 
(Essay)
4.8/5  (31)
(31)
Below is the mass spectrum of an unknown hydrocarbon. In addition, this hydrocarbon shows characteristic absorption at 2100 cm-1 in its IR spectrum. Give the structure of this unknown. 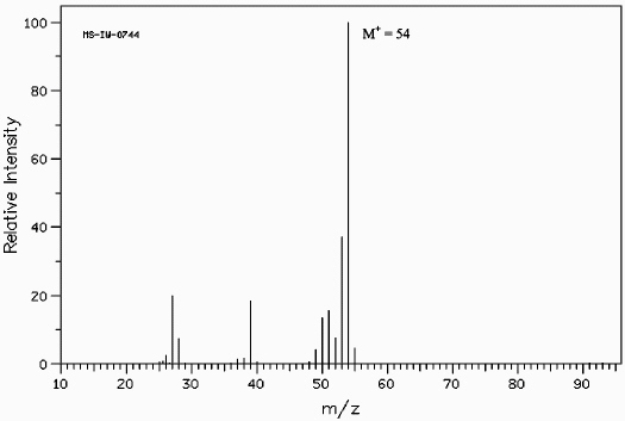 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
The formula weight of 54 corresponds to a molecular formula of C4H6, which has two degrees of unsaturation. Possible structures for this formula are:
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
The formula weight of 54 corresponds to a molecular formula of C4H6, which has two degrees of unsaturation. Possible structures for this formula are:
(Essay)
4.8/5  (42)
(42)
Showing 61 - 64 of 64
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)