Exam 13: Structure Determination
Exam 1: Structure and Bonding:acids and Bases40 Questions
Exam 2: Alkanes: The Nature of Organic Compounds47 Questions
Exam 3: Alkenes and Alkynes: the Nature of Organic Reactions37 Questions
Exam 4: Reactions of Alkenes and Alkynes44 Questions
Exam 5: Aromatic Compounds51 Questions
Exam 6: Sterechemistry at Tetrahedral Centers35 Questions
Exam 7: Organohalides: Nucleophilic Substitutions and Eliminations49 Questions
Exam 8: Alcohols, Phenols, Ethers, and Their Sulfur Analogs39 Questions
Exam 9: Aldehydes and Ketones: Nucleophilic Addition Reactions32 Questions
Exam 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions66 Questions
Exam 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions37 Questions
Exam 12: Amines31 Questions
Exam 13: Structure Determination64 Questions
Exam 14: Biomolecules: Carbohydrates41 Questions
Exam 15: Biomolecules: Amino Acids, Peptides, and Proteins43 Questions
Exam 16: Biomolecules: Lipids and Nucleic Acids37 Questions
Exam 17: The Organic Chemistry of Metabolic Pathways35 Questions
Select questions type
Instructions: For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
Number of signals: 
(Essay)
4.8/5  (38)
(38)
Treatment of tert-butyl alcohol with hydrogen chloride yields a mixture of tert-butyl chloride and 2-methylpropene. a) After chromatographic separation, how would you use NMR to help you decide which was which?
b) How would the NMR for the two compounds differ?
(Essay)
4.9/5  (30)
(30)
Which of the following does not involve the interaction of molecules with electromagnetic energy?
(Multiple Choice)
4.9/5  (39)
(39)
The following questions pertain to the charting of NMR spectra.xa0 MATCH a term to each description below.xa0
Correct Answer:
Premises:
Responses:
(Matching)
5.0/5  (48)
(48)
Which of the following statements best describes the base peak in a mass spectrum?
(Multiple Choice)
4.9/5  (43)
(43)
Instructions: Answer the following question(s) for the compound whose 1H NMR spectra is shown below.
C4H8O 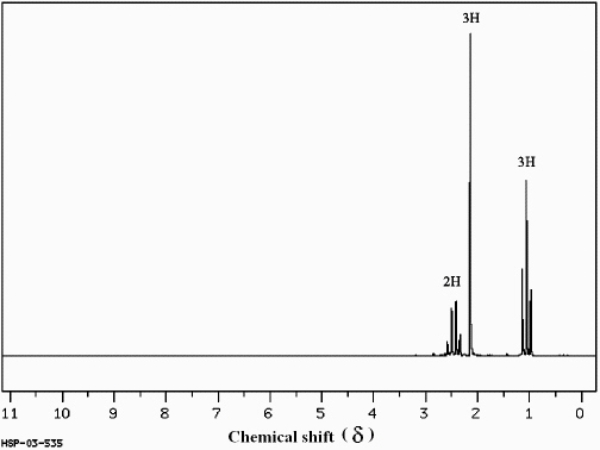 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. Propose a structure for this compound.
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
Refer to instructions. Propose a structure for this compound.
(Essay)
4.8/5  (51)
(51)
Instructions: The following questions pertain to the charting of NMR spectra. MATCH a term to each description below. Place the letter of the term in the blank to the left of the description.
- Refer to instructions. __________ The exact place on the chart at which a nucleus absorbs is called its __________.
(Multiple Choice)
4.9/5  (44)
(44)
Nuclear magnetic resonance spectroscopy provides information about a molecule's:
a. conjugated pi electron system
b. size and formula.
c. carbon-hydrogen framework.
d. functional groups.
(Short Answer)
4.8/5  (39)
(39)
Which structure of molecular formula C4H8Cl2 fits the 1H NMR and 13C NMR spectra shown below? 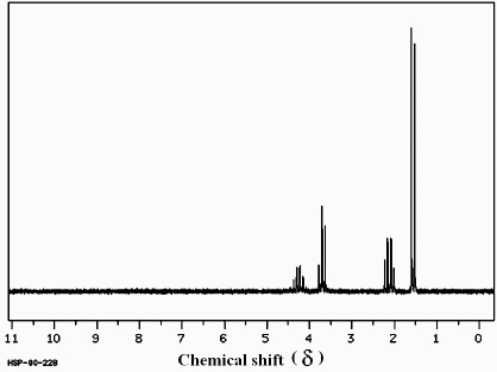
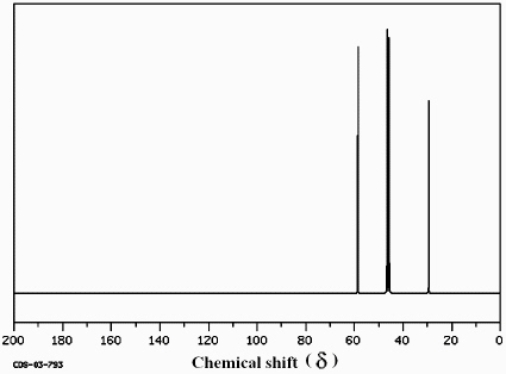 (Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Spectrum obtained from: SDBSWeb: http://www.aist.go.jp/RIODB/SDBS/)
(Multiple Choice)
4.9/5  (40)
(40)
A compound with the molecular formula C5H12O produces only two singlets in the 1H NMR spectrum.
a) Propose a structure for this compound.
b) How many signals would be present in the for this structure?
(Essay)
4.8/5  (34)
(34)
Instructions: Select the most reasonable formula for the compounds with the following mass spectral data. Refer to instructions. M+ at m/z = 216
(Multiple Choice)
4.8/5  (36)
(36)
Instructions: The following questions pertain to the charting of NMR spectra. MATCH a term to each description below. Place the letter of the term in the blank to the left of the description.
-Refer to instructions. __________ The calibration standard for 1H and 13C NMR is:
(Multiple Choice)
4.9/5  (44)
(44)
Cyclohexene and hex-2-yne both have the molecular formula, C6H10.
a) How would you use infrared spectroscopy to distinguish between the two compounds
b) How could the mass spectrum be used to distinguish between the two compounds?
(Essay)
4.7/5  (36)
(36)
Which of the following bonds undergoes stretching at the highest frequency?
(Multiple Choice)
4.8/5  (48)
(48)
Instructions: The following questions pertain to the charting of NMR spectra. MATCH a term to each description below. Place the letter of the term in the blank to the left of the description.
- Refer to instructions. __________ The NMR charts are calibrated using an arbitrary scale that is divided into __________ units.
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following combinations of peaks appears in the 1H NMR spectrum of 2-methylpropane?
(Multiple Choice)
4.8/5  (32)
(32)
Instructions: Predict the splitting patterns you would expect for each proton in the molecules below:
Predict: 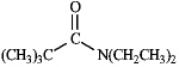
(Essay)
4.9/5  (34)
(34)
Which of the following compounds gives an infrared spectrum with a peak at strong 1730 cm-1?
(Multiple Choice)
4.8/5  (33)
(33)
When 2-bromopropane reacts with ethoxide ion, two products are formed; one is the product of SN2 substitution and the other is the product of E2 elimination. Write the structures of both products, and tell how they could be distinguished using IR spectroscopy.
(Essay)
5.0/5  (32)
(32)
Showing 41 - 60 of 64
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)