Exam 13: Structure Determination
Exam 1: Structure and Bonding:acids and Bases40 Questions
Exam 2: Alkanes: The Nature of Organic Compounds47 Questions
Exam 3: Alkenes and Alkynes: the Nature of Organic Reactions37 Questions
Exam 4: Reactions of Alkenes and Alkynes44 Questions
Exam 5: Aromatic Compounds51 Questions
Exam 6: Sterechemistry at Tetrahedral Centers35 Questions
Exam 7: Organohalides: Nucleophilic Substitutions and Eliminations49 Questions
Exam 8: Alcohols, Phenols, Ethers, and Their Sulfur Analogs39 Questions
Exam 9: Aldehydes and Ketones: Nucleophilic Addition Reactions32 Questions
Exam 10: Carboxylic Acids and Derivatives: Nucleophilic Acyl Substitution Reactions66 Questions
Exam 11: Carbonyl Alpha-Substitution Reactions and Condensation Reactions37 Questions
Exam 12: Amines31 Questions
Exam 13: Structure Determination64 Questions
Exam 14: Biomolecules: Carbohydrates41 Questions
Exam 15: Biomolecules: Amino Acids, Peptides, and Proteins43 Questions
Exam 16: Biomolecules: Lipids and Nucleic Acids37 Questions
Exam 17: The Organic Chemistry of Metabolic Pathways35 Questions
Select questions type
Instructions: For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
Number of signals: 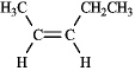
(Essay)
4.9/5  (38)
(38)
Which of the following compounds would produce the most downfield signal in a 13C NMR spectrum? 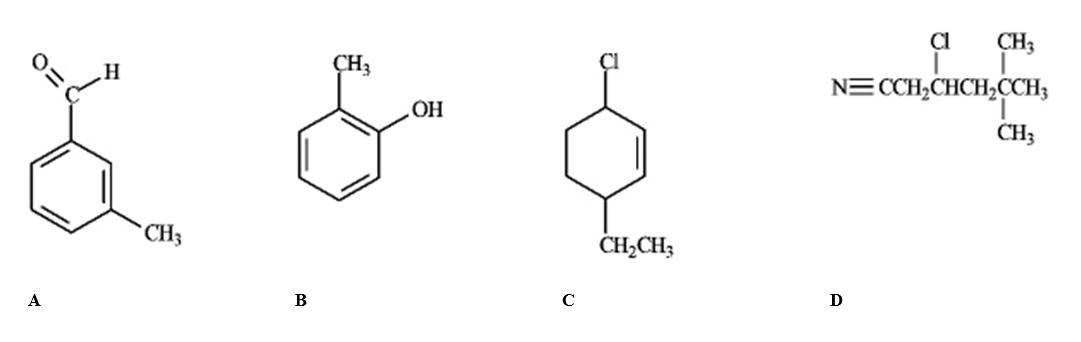
(Multiple Choice)
4.9/5  (34)
(34)
Circle any of the following compounds that would be a candidate to produce a UV absorption spectrum. 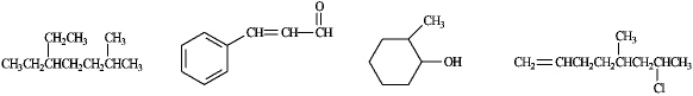
(Essay)
4.9/5  (33)
(33)
Instructions: Refer to the structure of 3-methylbutan-2-one below to answer the following question(s). 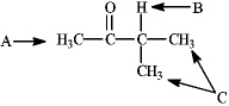 Refer to instructions. What is the splitting pattern for the hydrogens in 3-methylbutan-2-one labeled A, B, and C, respectively?
Refer to instructions. What is the splitting pattern for the hydrogens in 3-methylbutan-2-one labeled A, B, and C, respectively?
(Multiple Choice)
4.9/5  (37)
(37)
Consider the compound below: 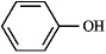 a) At what approximate positions might the compound show IR absorptions?
b) How would this spectrum differ from that of cyclohexand?
a) At what approximate positions might the compound show IR absorptions?
b) How would this spectrum differ from that of cyclohexand?
(Essay)
4.8/5  (33)
(33)
Which of the following compounds gives an infrared spectrum with a peak at ~1750 cm-1, but no significant peaks at 3000-3500 cm-1 or 1050-1250 cm-1?
(Multiple Choice)
4.7/5  (37)
(37)
Which feature in the 1H NMR spectrum provides information about the number of neighboring protons of each proton in the compound?
(Multiple Choice)
4.9/5  (34)
(34)
A 3.42 ´ 10-5 M solution of dibenzalacetone in ethanol produced an absorbance of 0.753 in a 1.00 cm cell. Based on this data, what is e for this compound?
(Multiple Choice)
5.0/5  (47)
(47)
Instructions: Refer to the structure of 3-methylbutan-2-one below to answer the following question(s). 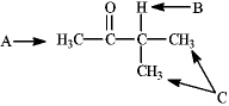 Refer to instructions. What is the ratio of peak areas upon integration of the spectrum for A, B, and C respectively?
Refer to instructions. What is the ratio of peak areas upon integration of the spectrum for A, B, and C respectively?
(Multiple Choice)
4.8/5  (38)
(38)
Instructions: Match a structure from the list below to the following IR spectra.
A.
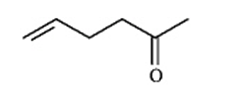 B.
B.
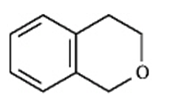 C.
C.
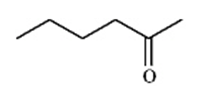 D.
D.
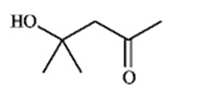 E.
E.
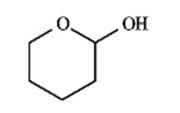 F.
F.
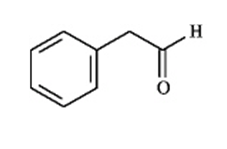
(Essay)
4.8/5  (39)
(39)
Which of the following compounds gives an infrared spectrum with peaks at 3000-3500 cm-1 and ~1750 cm-1?
(Multiple Choice)
4.8/5  (46)
(46)
Instructions: For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
Number of signals: 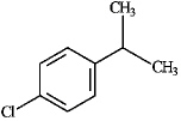
(Essay)
4.7/5  (36)
(36)
Which of the following bonds gives rise to a strong absorbance near 1700 cm-1 in the infrared spectrum?
(Multiple Choice)
5.0/5  (39)
(39)
The amount of energy in electromagnetic radiation is related to the frequency and wavelength of the radiation. High energy radiation, like gamma rays, is of:
(Multiple Choice)
4.8/5  (40)
(40)
A compound with the molecular formula C6H4ClBr produces only two doublets in the 1H NMR spectrum.
a) Propose a structure for this compound.
b) How many signals would be present in the for this structure?
(Essay)
4.9/5  (34)
(34)
Which of the following combinations of peaks appears in the 1H NMR spectrum of diethyl ether, CH3CH2OCH2CH3?
(Multiple Choice)
4.9/5  (41)
(41)
At what approximate positions might the compound below show IR absorptions? 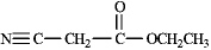
(Essay)
4.9/5  (46)
(46)
Instructions: For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
Number of signals: 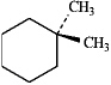
(Essay)
4.9/5  (37)
(37)
Which of the following compounds would have the longest lmax in the UV region of the electromagnetic spectrum? 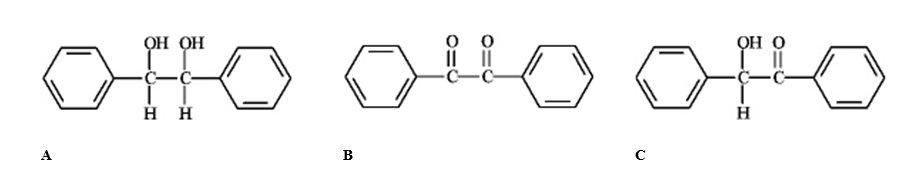
(Multiple Choice)
4.7/5  (40)
(40)
Showing 21 - 40 of 64
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)