Exam 9: Molecular Geometry and Bonding Theories
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Which of the following shows the orientation of the net dipole for OCS?
(Multiple Choice)
4.9/5  (31)
(31)
Arrange the interactions between pairs of electrons in order of increasing strength.
(Multiple Choice)
4.9/5  (32)
(32)
In 1995 a Japanese cult attacked the Tokyo subway system with the nerve gas Sarin, which focused world attention on the dangers of chemical warfare agents. The connectivity of atoms in the Sarin molecule is shown below. Complete the Lewis structure by adding bonds and lone pairs as necessary. Assign formal charges to the P and O atoms, and determine the local molecular geometry around the central oxygen atom. 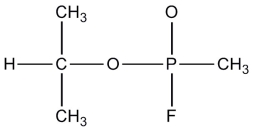
(Essay)
4.8/5  (32)
(32)
Which of the following statements about bonds between two carbon atoms is/are correct? (I) Bond strength increases as more electrons are shared between the atoms.
(II) Bond strength increases as the overlap between atomic orbitals increases.
(III) Hybrid orbitals are used to describe (account for) the geometry (bond angles) around each carbon atom.
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following has a central atom with the same hybridization as the carbon in formaldehyde (H2CO)?
(Multiple Choice)
4.8/5  (32)
(32)
Which one of the following molecules has a bond order of 3?
(Multiple Choice)
4.9/5  (38)
(38)
Using the energy level diagram below, determine the bond order of the PO molecule? 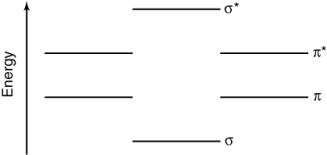
(Multiple Choice)
4.9/5  (35)
(35)
A Lewis structure of aspirin without the nonbonding electrons is shown in the figure below. Identify the hybridization of the C1 and C2 atomic orbitals. Arrange the bonds (B2-B5) in order of increasing length. 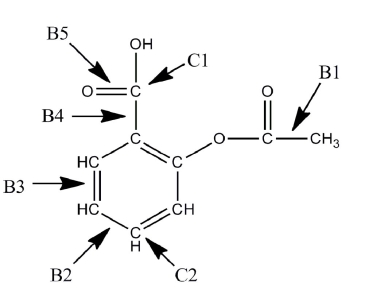
(Essay)
4.7/5  (32)
(32)
Which statement regarding a pi bond between two carbon atoms is correct?
(Multiple Choice)
4.9/5  (39)
(39)
Identify the local molecular geometry and hybridization at atoms a and b in Ritalin, which is a drug used to treat attention deficit hyperactivity disorder. In the line drawing shown below, the hydrogen atoms bonded to carbon are not shown explicitly. 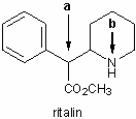
(Essay)
4.7/5  (37)
(37)
In VSEPR theory, molecular geometry is determined by __________
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following compounds has a central atom with the same hybridization as PCl5?
(Multiple Choice)
4.9/5  (39)
(39)
Describe the valence bond picture of bonding in ethylene, which is shown below. Identify the number of valence electrons, the number of pi bonds, the number of sigma bonds, and the hybridization of the carbon atomic orbitals. 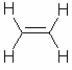
(Essay)
4.8/5  (35)
(35)
Which type of molecular orbital has maximum electron density above and below the internuclear axis but zero density in a plane perpendicular to the internuclear axis?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following has a central atom with the same hybridization as the oxygen in water?
(Multiple Choice)
4.8/5  (24)
(24)
Showing 21 - 40 of 101
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)