Exam 9: Molecular Geometry and Bonding Theories
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Boron nitride is being investigated in frontier research directed at producing novel electronic devices. If you used the following energy level diagram for the molecular orbitals of boron nitride, BN, what would you predict? 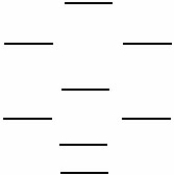 (I) Boron nitride is diamagnetic.
(II) Boron nitride has a bond order of 2.
(III) Boron nitride is paramagnetic.
(IV) The bond in BN- is stronger than the bond in BN.
(I) Boron nitride is diamagnetic.
(II) Boron nitride has a bond order of 2.
(III) Boron nitride is paramagnetic.
(IV) The bond in BN- is stronger than the bond in BN.
(Multiple Choice)
4.9/5  (39)
(39)
Draw a structure showing the geometry of the molecule in the following group that is nonpolar: HF; NH3; BF3; CHCl3; CO.
(Essay)
4.7/5  (29)
(29)
Which statement regarding a pi bond between two carbon atoms is correct?
(Multiple Choice)
4.7/5  (39)
(39)
Identify the hybridization of the atomic orbitals on carbon atoms labeled 1, 2, and 3. The hydrogen atoms and lone pair electrons are not shown in the diagram. 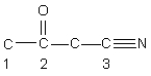
(Essay)
4.8/5  (34)
(34)
Which electron-pair geometry has the lowest electron-electron repulsive forces?
(Multiple Choice)
5.0/5  (35)
(35)
Identify the hybridization of atomic orbitals for atoms N1, N2, C1, C2, and C3 in caffeine, which is shown below. Explain why you think this molecule is planar or nonplanar. 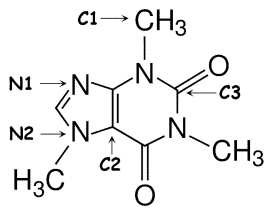
(Essay)
4.8/5  (36)
(36)
Which of the following compounds has a square pyramid shape?
(Multiple Choice)
4.9/5  (39)
(39)
Which type of molecular orbital contains a node perpendicular to the axis connecting two atomic nuclei?
(Multiple Choice)
4.7/5  (38)
(38)
Which of the following molecules has a bond order that is less than one?
(Multiple Choice)
4.9/5  (35)
(35)
Which one of the following statements regarding molecular orbitals for diatomic molecules is not correct?
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following compounds has the same molecular shape as CCl3H?
(Multiple Choice)
4.9/5  (36)
(36)
Use energy levels of diatomic molecules derived from molecular orbital theory to predict the magnetic properties of the oxygen molecule O2 and the peroxide anion O22-.
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following compounds has the largest dipole moment?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 61 - 80 of 101
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)