Exam 9: Molecular Geometry and Bonding Theories
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Which type of molecular orbital is used to describe a buildup of electron density along the axis connecting two atomic nuclei to form a bond?
(Multiple Choice)
4.7/5  (34)
(34)
Carbonyl dihalides (COX2 with X = I, Cl, or Br) are irritants and can cause blistering of tissue. Their reactivity with tissue is influenced by their polarity. Place these compounds in order of decreasing polarity and explain your reasoning.
(Essay)
4.9/5  (41)
(41)
For the molecule CH3CHCHCH3, the local molecular geometry around a carbon atom at the end and its hybridization are ___________
(Multiple Choice)
4.9/5  (29)
(29)
Which type of molecular orbital is used to describe electron density building up above and below the internuclear axis to form a bond?
(Multiple Choice)
4.7/5  (31)
(31)
Identify the molecular structure of the molecular anion, 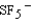 , and the hybridization of the S atomic orbitals.
, and the hybridization of the S atomic orbitals.
(Short Answer)
4.8/5  (40)
(40)
The amide structure is the fundamental linking unit in proteins. Two partial Lewis structures for formamide are shown below. These structures do not show the lone pair electrons or the formal charges. Experiment shows that formamide is planar, so which is the better representation of the electronic structure, I or II? Is this conclusion consistent with the formal charges on the atoms? Explain the rationale for your answers. 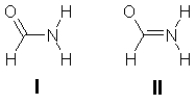
(Essay)
4.7/5  (44)
(44)
A Lewis structure of aspirin without the nonbonding electrons is shown in the figure below. Taking into account the nonbonding electrons, identify the hybridization of the atomic orbitals for the following atoms: O1, O2, and O3. Identify the bond angles around C1, C2, and O3. 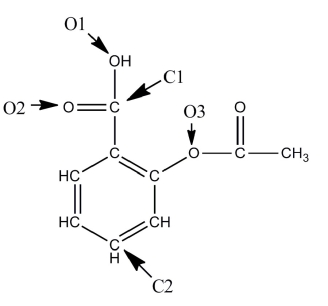
(Essay)
4.8/5  (36)
(36)
Use MO theory to predict the bond orders of the following molecular ions. Which one do you predict does not exist?
(Multiple Choice)
4.8/5  (33)
(33)
Use energy levels of diatomic molecules derived from molecular orbital theory to predict the bond order of the oxygen molecule O2 and the peroxide anion O22-.
(Multiple Choice)
5.0/5  (36)
(36)
Which of the following compounds has the same shape as SO2?
(Multiple Choice)
4.9/5  (49)
(49)
Identify the hybridization of atomic orbitals for atoms a, b, and c in the structure below of acetaminophen, which is the active ingredient in the analgesic Tylenol. 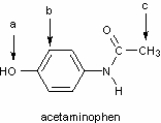
(Short Answer)
4.7/5  (31)
(31)
Both cyclohexane (C6H12) and benzene (C6H6) have the carbon atoms forming a six-member ring. In a valence bond picture of the C - C bonds, _____ hybrid orbitals would overlap for cyclohexane, and _____ hybrid orbitals would overlap for benzene.
(Multiple Choice)
5.0/5  (41)
(41)
What type of hybridization is needed to describe the bonding in a T-shaped molecule?
(Multiple Choice)
5.0/5  (41)
(41)
Which of the following diagrams shows the correct orientation of the dipole in sulfur dioxide?
(Multiple Choice)
4.8/5  (29)
(29)
Which statement regarding a sigma bond between two carbon atoms is not correct?
(Multiple Choice)
4.7/5  (42)
(42)
For the molecule CH3CHCHCH3, the local molecular geometry around the second carbon atom from the end and its hybridization are ___________
(Multiple Choice)
4.8/5  (31)
(31)
Showing 41 - 60 of 101
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)