Exam 4: Solution Chemistry and the Hydrosphere
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Write the balanced molecular equation and the net ionic equation for the reaction of solid magnesium hydroxide with a solution of sulfuric acid.
(Essay)
4.8/5  (36)
(36)
If one regular antacid tablet contains 500 mg of solid CaCO3 (100 g/mol), how many mL of 1.0 M stomach acid (HCl) could it neutralize?
(Multiple Choice)
4.7/5  (31)
(31)
Arsenic, which is toxic, can be removed from drinking water by precipitation as the sulfide. Write the net ionic equation for the precipitation of As3+ using sodium sulfide, Na2S.
(Essay)
4.9/5  (37)
(37)
Controlling the concentration of ammonia is one of the biggest problems in maintaining a healthy fish aquarium. Ammonia, which is highly toxic to fish because it interferes with their uptake of oxygen, is formed from wastes excreted by the fish, uneaten food, and decaying plants. Beneficial bacteria oxidize the ammonia to nitrate, which is not highly toxic to fish in low to moderate levels. Complete and balance the following reaction equation describing the oxidation of ammonia to nitrate in basic solution.
NH3(aq) + O2(aq) NO3-
(Essay)
4.8/5  (48)
(48)
Which contains more solute particles: a 0.10 M aqueous solution of methanol (CH3OH) or a 0.10 M aqueous solution of salt (NaCl)?
(Multiple Choice)
4.8/5  (46)
(46)
If 1.0 L of 1.0 M HCl spilled and needed to be neutralized, how many grams of the solid sodium carbonate (Na2CO3, 106 g/mol) would be required?
(Multiple Choice)
4.9/5  (38)
(38)
Two phosphoric acid molecules can combine to make pyrophosphoric acid and another molecule as shown here. The pyrophosphoric acid molecule is a phosphoester and is a structural element in the ADP/ATP metabolic energy system. What molecule is represented by the question mark in the structural reaction equation below? 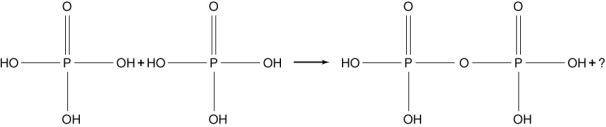
(Multiple Choice)
4.9/5  (37)
(37)
Most chloride salts are soluble. Identify an exception to this generalization.
(Multiple Choice)
4.7/5  (39)
(39)
A standard adult dose of a cough suppressant is 25.0 mL. This portion contains 50.0 mg of the active ingredient. A pediatrician suggests that this medication can be given to children over the age of 6, but only if it is diluted so the dose is 5.00 mg in 10.0 mL. How many mL of the adult medicine would you need to use to make 100.0 mL of the child-strength cough suppressant?
(Short Answer)
4.8/5  (38)
(38)
According to the label on a bottle of concentrated hydrochloric acid, the contents are 35% HCl by mass and have a density of 1.2 g/mL. What is the molarity of commercial concentrated hydrochloric acid?
(Short Answer)
4.7/5  (33)
(33)
Milk of Magnesia is a liquid antacid. The active ingredient is magnesium hydroxide. What volume of 0.15 M stomach acid (HCl) could be neutralized by a dose of 10.0 mL containing 820 mg of the active ingredient?
(Short Answer)
5.0/5  (31)
(31)
If 100 mL of 3.0 M solution were diluted to 250 mL, what would the concentration be?
(Multiple Choice)
5.0/5  (40)
(40)
Sodium fluoride is added to drinking water in some municipalities to protect teeth against cavities. The idea is to convert hydroxyapatite, Ca10(PO4)6(OH)2, into more stable fluorapatite, Ca10(PO4)6F2. Express a flouride concentration of 5 mg/L molarity.
(Essay)
4.9/5  (34)
(34)
Methane (CH4) is a suitable fuel for burning because it is readily oxidized by oxygen gas, forming carbon dioxide and water. Hydrogen is also a good fuel for burning with oxygen gas, forming water as a product. What change in oxidation number always accompanies oxidation?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 81 - 98 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)