Exam 4: Solution Chemistry and the Hydrosphere
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Hydroxyapatite, [Ca5(PO4)3(OH)], the major component of tooth enamel, is attacked and decomposed by acids more readily than fluorapatite, [Ca5(PO4)3F]. Which one of the following reactions is analogous to the reaction of hydroxyapatite?
(Multiple Choice)
4.7/5  (40)
(40)
Chlorine, ozone, and chlorine dioxide are all common disinfectants for municipal water purification. What chemical property do they all share?
(Multiple Choice)
4.8/5  (25)
(25)
A reaction that takes place between dissolved barium nitrate and dissolved sodium sulfate results in formation of solid barium sulfate. Which equation describes this reaction?
(Multiple Choice)
5.0/5  (38)
(38)
Ammonia (NH3) is a weak base that reacts with a strong acid to form the ammonium ion, NH4+. If 5.00 mL of a solution of an ammonia cleaner is titrated directly with 42.6 mL of 0.5000 M HCl, what is the concentration of the NH3 in solution? (Assume that the ammonia is the only solute that reacts with the acid.)
(Multiple Choice)
4.8/5  (36)
(36)
The half-reaction for the reduction of molecular oxygen to water is __________
(Multiple Choice)
4.8/5  (35)
(35)
What is the molar concentration of sodium in a 200.0 mL solution prepared from 1.223 g of sodium phosphate (Na3PO4, 163.9 g/mol), which is a cleaning agent, food additive, and stain remover?
(Multiple Choice)
4.9/5  (42)
(42)
How many grams of solid magnesium chloride, MgCl2, are needed to make 250 mL of 0.50 M solution?
(Multiple Choice)
4.8/5  (42)
(42)
Concentrated sulfuric acid contains 4 g of water for every 100 g of solution. The solvent is __________
(Multiple Choice)
4.8/5  (42)
(42)
Potassium is a very reactive metal. Many of its compounds are not very reactive. For example, some class B fire extinguishers contain potassium bicarbonate as a dry powder for use in extinguishing burning liquids. Why is there such a difference in the reactivity of potassium in the bicarbonate salt and potassium that is a combustible metal?
(Multiple Choice)
4.8/5  (40)
(40)
In which compound does chlorine have an oxidation number of +5?
(Multiple Choice)
4.8/5  (39)
(39)
How many grams of sodium chloride, NaCl, are needed to make up 1.00 L of a solution that is 2.00 M ?
(Multiple Choice)
4.8/5  (31)
(31)
Which picture best represents an atomic-level view of a nonelectrolyte solution (water molecules not shown)?
(Multiple Choice)
4.9/5  (38)
(38)
The carbon cycle characterizes the cyclic transformations of the element carbon through the environment and biomass. Some of the important species in this cycle are CO2, CO32-, C6H12O6, and CH4. Which of these compounds would undergo complete oxidation by O2 to give the largest change in oxidation number?
(Multiple Choice)
4.7/5  (32)
(32)
Some microorganisms living under anaerobic conditions extract oxygen from sulfate ions producing hydrogen sulfide gas, which produces a distinctive rotten-egg odor. Write the balanced net ionic equation for this reaction under acidic conditions.
(Essay)
4.8/5  (44)
(44)
A food chemist determined the concentration of acetic acid in a sample of apple cider vinegar. The density of the sample was 1.01 g/mL. The volume of 1.215 M NaOH required to titrate a 25.00 mL sample of the vinegar was 19.85 mL. What is (a) the molar concentration, and (b) the percent by mass of acetic acid in this vinegar? (CH3COOH, 60.05 g/mol)
(Short Answer)
4.9/5  (35)
(35)
Water-soluble toxic chromium compounds are waste products of electroplating operations, but the chromium can be precipitated as Cr(OH)3 to remediate the water. How much 1.0 M NaOH solution is needed to remove the chromium from 100 L of a solution that is 0.001 M in Cr3+?
(Multiple Choice)
4.7/5  (35)
(35)
How many liters of 0.200 M NaOH solution are required to completely react with 1.00 L of 0.100 M HCN solution to produce sodium cyanide and water?
(Multiple Choice)
4.9/5  (38)
(38)
Identify the acid in the following acid-base reaction. PbCO3(s) + H2SO4(aq) PbSO4(s) + CO2(g) + H2O( 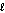 )
)
(Multiple Choice)
4.8/5  (29)
(29)
Showing 21 - 40 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)