Exam 4: Solution Chemistry and the Hydrosphere
Exam 1: Matter, Energy, and the Origins of the Universe77 Questions
Exam 2: Atoms, Ions, and Compounds102 Questions
Exam 3: Chemical Reactions and Earths Composition97 Questions
Exam 4: Solution Chemistry and the Hydrosphere98 Questions
Exam 5: Thermochemistry101 Questions
Exam 6: Properties of Gases: the Air We Breathe106 Questions
Exam 7: Electrons in Atoms and Periodic Properties104 Questions
Exam 8: Chemical Bonding and Climate Change104 Questions
Exam 9: Molecular Geometry and Bonding Theories101 Questions
Exam 10: Forces Between Ions and Molecules100 Questions
Exam 11: Solutions and Their Colligative Properties92 Questions
Exam 12: The Chemistry of Solids128 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, and Materials112 Questions
Exam 14: Thermodynamics: Spontaneous Processes, Entropy, and Free Energy79 Questions
Exam 15: Chemical Kinetics128 Questions
Exam 16: Chemical Equilibrium105 Questions
Exam 17: Equilibrium in the Aqueous Phase156 Questions
Exam 18: The Colorful Chemistry of Metals114 Questions
Exam 19: Electrochemistry and the Quest for Clean Energy103 Questions
Exam 20: Biochemistry: the Compounds of Life109 Questions
Exam 21: Nuclear Chemistry108 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Solutions of hydrochloric acid, sulfuric acid, and phosphoric acid with equal concentrations and volumes are neutralized by a solution of sodium hydroxide. If it takes 25.0 mL of the sodium hydroxide solution to neutralize the hydrochloric acid, what volume of the sodium hydroxide solution does it take to neutralize each of the other two solutions? Explain.
(Essay)
4.9/5  (41)
(41)
Identify the solution below that will conduct electricity the best, and explain why you picked it.
(a) 1.0 M NaCl, (b) 1.2 M KCl, (c) 1.0 M Na2SO4, (d) 0.75 M LiCl
(Essay)
4.8/5  (36)
(36)
Diluting 1.0 mL of a 1.0 M solution to 1000 mL results in a solution that is 0.001 M. Repeating this 1000-fold dilution process five more times results in a concentration of __________
(Multiple Choice)
4.9/5  (37)
(37)
Chalk contains calcium carbonate. What would be the best solution for cleaning a sidewalk that a preschool class covered with smiling suns, flowers, birds, rainbows, and houses using sidewalk chalk?
(Multiple Choice)
4.8/5  (37)
(37)
Tube worms that survive near geothermal vents of hydrogen sulfide rely on bacteria living inside them to obtain energy by the oxidation of H2S to SO42-. What is the overall change in the oxidation number of sulfur for this reaction?
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following ionic compounds is insoluble in water?
(Multiple Choice)
4.8/5  (29)
(29)
Silver salts are used in black-and-white photography. Owing to the value of silver, the ions left in developing solutions are often collected by precipitation reactions for recycling. A 4.5 L jug of a developing solution was treated with an excess of sodium chloride, and a precipitate containing 2.86 g of dry silver chloride was collected. What was the molar concentration of the silver ions in the developing solution? The net ionic equation is Ag+(aq) + Cl-(aq) AgCl(s)
(Multiple Choice)
4.8/5  (30)
(30)
A typical adult body contains about 6.0 L of blood. The hemoglobin content is about 16 g per 100.0 mL of blood. The molar mass of hemoglobin is approximately 64,000 g/mol. (a) What is the molar concentration of hemoglobin in blood, and (b) how many moles of hemoglobin are present in a typical adult?
(Essay)
4.9/5  (39)
(39)
Hard water contains Mg2+ and Ca2+ ions and tends to form a ring in a bathtub due to its reaction with the soluble anions in soap. The formation of this insoluble material is an example of __________
(Multiple Choice)
4.9/5  (39)
(39)
In the following reactions, which element is oxidized? Cu + FeSO4 Fe + CuSO4
(Multiple Choice)
4.8/5  (39)
(39)
Sodium metal easily loses an electron to form the sodium ion. In this process, sodium is __________
(Multiple Choice)
4.8/5  (45)
(45)
Determine the molar concentration of an aqueous solution of lead(II) nitrate solution that is 26 ppb (parts per billion) Pb(NO3)2.
(Multiple Choice)
4.8/5  (41)
(41)
Why is an aqueous solution of table salt (NaCl) a good conductor of electricity but a solution containing an equal molar concentration of table sugar (C12H22O11) is not?
(Essay)
4.8/5  (34)
(34)
If there are 0.505 g of NaCl left in a beaker that originally contained 75.0 mL of saltwater, what must have been the concentration of the original solution?
(Multiple Choice)
4.9/5  (40)
(40)
The proof of liquor is defined as the percentage of ethanol it contains times two. If vodka is 80 proof, what is the solvent in vodka?
(Multiple Choice)
4.9/5  (30)
(30)
Polymers with ionic functional groups have been developed for removal of ions from water. One example is Amberlite. One form of Amberlite has the structure shown below, where the -SO3H groups act like a weak acid. What type of ions will this polymer attract? 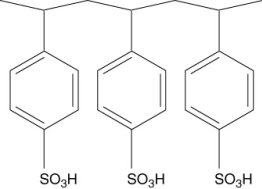
(Multiple Choice)
4.9/5  (37)
(37)
In a spontaneous oxidation-reduction reaction between aluminum and silver ion, the aluminum(III) ion and solid silver are formed. If 0.1 mol of aluminum is consumed in this reaction, how much silver will be produced?
(Multiple Choice)
4.8/5  (35)
(35)
A 500 mg dietary supplement of L-lysine (an amino acid, 146.2 g/mol) required 68.4 mL of 0.100 M NaOH to reach the end point. How many protons were removed for each L-lysine molecule in this titration?
(Multiple Choice)
4.8/5  (36)
(36)
Showing 41 - 60 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)