Exam 8: Applications of Aqueous Equilibria
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
In the titration of 250.0 mL of 0.20 M H3PO4 with 0.10 M NaOH, the pH of the solution after the addition of some NaOH is 4.66. Which of the following phosphate-containing species is present in the largest amount? For H3PO4, Ka1 = 7.5 10-3, Ka2 = 6.2 10-8, and Ka3 = 4.8 10-13.
(Multiple Choice)
4.8/5  (37)
(37)
A 135.0-mL sample of a 0.25 M solution of H3PO4 is titrated with 0.12 M NaOH. What volume of base must be added to reach the third equivalence point?
(Multiple Choice)
4.8/5  (46)
(46)
You dissolve 1.24 g of an unknown diprotic acid in 220.0 mL of H2O. This solution is just neutralized by 6.44 mL of a 1.25 M NaOH solution. What is the molar mass of the unknown acid?
(Multiple Choice)
4.9/5  (46)
(46)
Calculate the minimum concentration of 100.0 mL of sodium sulfate that is required to form a precipitate with 100.0 mL of 1.00 10-2 M calcium nitrate. Ksp for CaSO4 is 6.1 10-5.
(Multiple Choice)
4.9/5  (37)
(37)
Consider a 100.0-mL sample of a 0.10 M H2A (Ka1 = 1.3 *10-3 , Ka2 = 3.1 * 10-6) with 0.10 M NaOH. Determine the pH when 0 mL, 50.0 mL, 100.0 mL, and 150.0 mL of 0.10 M NaOH are added.
(Essay)
4.7/5  (34)
(34)
Determine the pH of a solution prepared by mixing the following: 25.0 mL of 0.200 M HCl
75)0 mL of 0.100 M NaOH
50)0 mL of 0.200 M NaH2PO4
For H3PO4 Ka1 = 7.5 10-3, Ka2 = 6.2 10-8, and Ka3 = 4.8 10-13.
(Multiple Choice)
4.9/5  (31)
(31)
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
-After 200.0 mL of 0.200 M NaOH is added
(Multiple Choice)
4.7/5  (41)
(41)
Consider the titration of 200.0 mL of a 0.100 M solution of the weak acid H2A with 0.200 M NaOH. The first equivalence point is reached after 100.0 mL of 0.200 M NaOH has been added, and the pH is 6.27. The pH after 65.0 mL of 0.200 M NaOH has been added, the pH is 4.95. Calculate the value of Ka2 for H2A.
(Multiple Choice)
4.8/5  (40)
(40)
Calculate the molar concentration of uncomplexed Zn2+ in a solution that contains 0.20 mol of Zn(NH3)42+ per liter. The overall Kf for Zn(NH3)42+ is 3.8 109.
(Multiple Choice)
4.8/5  (40)
(40)
For 110.0 mL of a buffer that is 0.40 M in HOCl and 0.52 M in NaOCl, what is the pH after 11.2 mL of 1.1 M NaOH is added? Ka for HOCl = 3.5 10-8. (Assume the volumes are additive.)
(Multiple Choice)
4.8/5  (29)
(29)
The solubility of AgCl in water is _____ the solubility of AgCl in strong acid at the same temperature.
(Multiple Choice)
4.9/5  (32)
(32)
Calculate [H+] in a solution that is 0.24 M in NaF and 0.46 M in HF. (Ka = 7.2 10-4)
(Multiple Choice)
4.9/5  (39)
(39)
Consider the following information about the diprotic acid ascorbic acid (H2As for short, molar mass = 176.1). 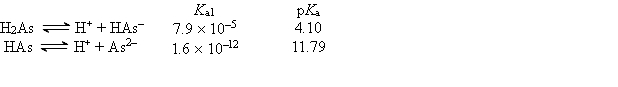 The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 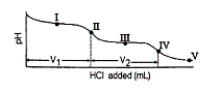 -Which of the following is a major species present at point IV?
-Which of the following is a major species present at point IV?
(Multiple Choice)
4.8/5  (37)
(37)
How much solid NaCN must be added to 1.0 L of a 0.5 M HCN solution to produce a solution with pH 7.0? Ka = 6.2 10-10 for HCN.
(Multiple Choice)
4.7/5  (39)
(39)
The solubility of Fe(OH)2 in water is 7.9 10-6 mol/L at 25° C. What is Ksp for Fe(OH)2 at 25° C?
(Multiple Choice)
4.9/5  (40)
(40)
In the titration of a weak acid HA with 0.100 M NaOH, the stoichiometric point is known to occur at a pH value of approximately 11. Which of the following indicators would be best to use to mark the endpoint of this titration?
(Multiple Choice)
4.9/5  (41)
(41)
2.42 10-3 g of BaSO4 can dissolve in 1.00 L of water. Calculate Ksp for this salt.
(Multiple Choice)
4.9/5  (33)
(33)
A 50.00-mL sample of 0.100 M KOH is titrated with 0.100 M HNO3. Calculate the pH of the solution after the 52.00 mL of HNO3 is added.
(Multiple Choice)
4.8/5  (38)
(38)
A solution containing 10. mmol of CO32- and 5.0 mmol of HCO3- is titrated with 1.0 M HCl.
What total volume of HCl must be added to reach the second equivalence point?
(Multiple Choice)
4.8/5  (42)
(42)
A student titrates an unknown weak acid, HA, to a pale pink phenolphthalein endpoint with 25.0 mL of 0.100 M NaOH. The student then adds 13.0 mL of 0.100 M HCl. The pH of the resulting solution is 4.7. Which of the following statements is true?
(Multiple Choice)
4.8/5  (42)
(42)
Showing 81 - 100 of 171
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)