Exam 8: Applications of Aqueous Equilibria
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
Explain why we cannot directly compare Ksp values in comparing solubilities of ionic solids with different numbers of ions.
(Essay)
4.8/5  (37)
(37)
A 200-mL solution contains 0.018 mol each of I-, Br-, and Cl-. When the solution is mixed with 200 mL of 0.24 M AgNO3, how much AgCl(s) precipitates out? 
(Multiple Choice)
4.8/5  (33)
(33)
After adding 25.0 mL of 0.100 M NaOH to 100.0 mL of 0.100 M weak acid (HA), the pH is found to be 5.90. Determine the value of Ka for the acid HA.
(Multiple Choice)
4.8/5  (35)
(35)
Equal volumes of 0.1 M HCl and 0.1 M HC2H3O2 are titrated with 0.1 M NaOH. Which of the following would be equal for both titrations?
(Multiple Choice)
4.8/5  (35)
(35)
Consider the titration of 200.0 mL of a 0.100 M solution of the weak acid H2A with 0.200 M NaOH. The first equivalence point is reached after 100.0 mL of 0.200 M NaOH has been added, and the pH is 6.27. The pH after 65.0 mL of 0.200 M NaOH has been added, the pH is 4.95. Calculate the value of Ka1 for H2A.
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following solutions will be the best buffer at a pH of 9.26? (Ka for HC2H3O2 is 1.8 10-5; Kb for NH3 is 1.8 10-5.)
(Multiple Choice)
4.9/5  (32)
(32)
You have a solution of 0.10 M Cl- and 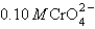 . You add 0.10 M silver nitrate dropwise into the solution. Ksp for Ag2CrO4 is 9.0 10-12 and for AgCl is1.6 10-10. Which of the following will precipitate first?
. You add 0.10 M silver nitrate dropwise into the solution. Ksp for Ag2CrO4 is 9.0 10-12 and for AgCl is1.6 10-10. Which of the following will precipitate first?
(Multiple Choice)
4.8/5  (33)
(33)
Explain how the solubility of an ionic solid at a constant temperature for a given ionic solid can vary.
(Essay)
4.8/5  (40)
(40)
11.8 mL of 0.30 M HCl is added to a 122.0-mL sample of 0.210 M HNO2 (Ka for HNO2 = 4.0 10-4). What is the equilibrium concentration of NO2- ions?
(Multiple Choice)
4.8/5  (37)
(37)
What is the solubility of Mg(OH)2 (Ksp = 8.9 10-12) in 1.0 L of a solution buffered (with large capacity) at pH 10.0?
(Multiple Choice)
4.9/5  (32)
(32)
Silver acetate (AgC2H3O2) is a sparingly soluble salt with Ksp = 1.9 10-3. Consider a saturated solution in equilibrium with the solid salt. Compare the effects on the solubility of adding to the solution either the acid HNO3 or the base NH3.
(Multiple Choice)
4.8/5  (41)
(41)
The two salts AgX and AgY have very similar solubilities in water. The salt AgX is much more soluble in acid than is AgY. What can be said about the relative strengths of the acids HX and HY?
(Multiple Choice)
4.8/5  (36)
(36)
Calculate the pH of a solution prepared by mixing 50 mL of a 0.10 M solution of HF with 25 mL of a 0.20 M solution of NaF. pKa of HF is 3.14.
(Multiple Choice)
4.8/5  (40)
(40)
The value of Ksp for AgI is 1.5 10-16. Calculate the solubility, in moles per liter, of AgI in a 0.36 M NaI solution.
(Multiple Choice)
4.8/5  (33)
(33)
You have 0.20 M HNO2 (Ka = 4.0 *10-4) and 0.20 M KNO2. You need 1.00 L of a buffered solution at a pH of 3.00. What volumes of each solution do you add together to make this buffered solution?
(Essay)
4.9/5  (38)
(38)
You are given a solution of the weak base Novocain, Nvc. Its pH is 11.00. You add to the solution a small amount of a salt containing the conjugate acid of Novocain, NvcH+. Which statement is true?
(Multiple Choice)
4.8/5  (31)
(31)
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 10-6, Ka2 = 1.0 10-10) is titrated with the following volumes of 1.00 M NaOH.
-0 mL of 1.00 M NaOH
(Multiple Choice)
4.8/5  (34)
(34)
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 10-6, Ka2 = 1.0 10-10) is titrated with the following volumes of 1.00 M NaOH.
-150.0 mL of 1.00 M NaOH
(Multiple Choice)
4.8/5  (37)
(37)
Given the following values of equilibrium constants: 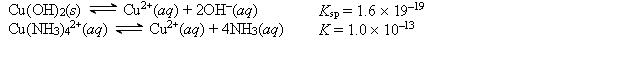 What is the value of the equilibrium constant for the following reaction? Cu(OH)2(s) + 4NH3(aq)
What is the value of the equilibrium constant for the following reaction? Cu(OH)2(s) + 4NH3(aq)  Cu(NH3)42+(aq) + 2OH-(aq)
Cu(NH3)42+(aq) + 2OH-(aq)
(Multiple Choice)
4.9/5  (30)
(30)
Solubility Products (Ksp)
BaSO4 1.5 10-9
CoS 5.0 10-22
PbSO4 1.3 10-8
AgBr 5.0 10-13
BaCO3 1.6 10-9
Which of the following compounds is the most soluble (in moles per liter)?
(Multiple Choice)
4.7/5  (38)
(38)
Showing 141 - 160 of 171
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)