Exam 8: Applications of Aqueous Equilibria
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
Which of the following compounds has the lowest solubility, in moles per liter, in water?
(Multiple Choice)
4.9/5  (45)
(45)
A 0.012-mol sample of Na2SO4 is added to 400 mL of each of two solutions. One solution contains 1.5 10-3 M BaCl2; the other contains 1.5 10-3 M CaCl2. Ksp for BaSO4 = 1.5 10-9 and Ksp for CaSO4 = 6.1 10-5. Which of the following statements is true?
(Multiple Choice)
5.0/5  (34)
(34)
One milliliter (1.00 mL) of acid taken from a lead storage battery is pipetted into a flask. Water and phenolphthalein indicator are added, and the solution is titrated with 0.44 M NaOH until a pink color appears; 13.6 mL is required. Find, to within 5%, the number of grams of H2SO4 (formula weight = 98) present in 1 L of the battery acid.
(Multiple Choice)
4.9/5  (31)
(31)
Titrating 30.00 mL of a saturated calcium iodate solution requires 28.91 mL of a 0.092 M solution of Na2S2O3 according to the equation IO3- + 6S2O32- + 6H+ I- + 2S3O62- + 3H2O
Calculate Ksp for Ca(IO3)2.
(Multiple Choice)
4.8/5  (35)
(35)
Consider a solution consisting of the following two buffer systems: H2CO3  HCO3- + H+ pKa = 6.4
H2PO4-
HCO3- + H+ pKa = 6.4
H2PO4-  HPO42- + H+ pKa = 7.2
At pH 6.4, which one of the following is true of the relative amounts of acid and conjugate base present?
HPO42- + H+ pKa = 7.2
At pH 6.4, which one of the following is true of the relative amounts of acid and conjugate base present?
(Multiple Choice)
4.8/5  (30)
(30)
A solution is formed by mixing 50.0 mL of 10.00 M NaCN with 50.0 mL of 2.0 10-3 M CuNO3. Cu(I) forms complex ions with cyanide as follows: ![A solution is formed by mixing 50.0 mL of 10.00 M NaCN with 50.0 mL of 2.0 <font face=symbol></font> 10<sup>-3</sup> M CuNO<sub>3</sub>. Cu(I) forms complex ions with cyanide as follows: Calculate the following concentrations at equilibrium: -[Cu(CN)<sub>2</sub><sup>-</sup>]](https://storage.examlex.com/TB6420/11eaaf8d_c168_297e_892c_296022b8a32c_TB6420_00_TB6420_00_TB6420_00.jpg) Calculate the following concentrations at equilibrium:
-[Cu(CN)2-]
Calculate the following concentrations at equilibrium:
-[Cu(CN)2-]
(Multiple Choice)
4.9/5  (37)
(37)
How many moles of HCl need to be added to 150.0 mL of 0.50 M NaZ to have a solution with a pH of 6.50? (Ka of HZ is 2.3 10-5.) Assume negligible volume of the HCl.
(Multiple Choice)
4.8/5  (36)
(36)
Chromate ion is added to a saturated solution of Ag2CrO4 to reach 0.10 M CrO42-. Calculate the final concentration of silver ion at equilibrium (Ksp for Ag2CrO4 is 9.0 10-12).
(Multiple Choice)
4.8/5  (32)
(32)
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 10-6, Ka2 = 1.0 10-10) is titrated with the following volumes of 1.00 M NaOH.
-250.0 mL of 1.00 M NaOH
(Multiple Choice)
4.8/5  (33)
(33)
Calculate the pH of the final solution obtained by mixing the following solutions. For HCN, Ka = 6.2 * 10-10.
50.0 mL of 0.10 M HNO3
60.0 mL of 0.20 M Ba(OH)2
95.0 mL of 0.20 M HClO4
195.0 mL of 1.0 *10-4 M HCN
(Short Answer)
4.9/5  (39)
(39)
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 10-6, Ka2 = 1.0 10-10) is titrated with the following volumes of 1.00 M NaOH.
-200.0 mL of 1.00 M NaOH
(Multiple Choice)
5.0/5  (36)
(36)
The salt AgCl is ________ soluble in strong acid solution than in water.
(Multiple Choice)
4.8/5  (42)
(42)
The concentration of OH- in a saturated solution of Mg(OH)2 is 3.6 10-4 M. What is Ksp for Mg(OH)2?
(Multiple Choice)
4.8/5  (36)
(36)
A solution contains 0.34 M HA (Ka = 2.0 10-7) and 0.17 M NaA. Calculate the pH after 0.05 mol of NaOH is added to 1.00 L of this solution.
(Multiple Choice)
4.9/5  (41)
(41)
Methyl orange is an indicator with a Ka of 1 10-4. Its acid form, HIn, is red, while its base form, In-, is yellow. At pH 6.0, the indicator will be
(Multiple Choice)
4.7/5  (27)
(27)
In the titration of 100.0 mL of a 0.200 M solution of H2A (Ka1 = 1.0 10-5, Ka2 = 1.0 10-8), what volume of 0.400 M NaOH must be added to reach a pH of 5.00?
(Multiple Choice)
4.8/5  (46)
(46)
Given the following Ksp values 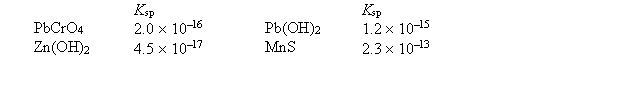 which statement about solubility, in moles per liter, in water is correct?
which statement about solubility, in moles per liter, in water is correct?
(Multiple Choice)
4.7/5  (40)
(40)
Differentiate between the equivalence point and the endpoint in an acid-base titration.
(Essay)
4.8/5  (35)
(35)
A 50.0-mL sample of 2.0 10-4 M CuNO3 is added to 50.0 mL of 4.0 M NaCN. Cu+ reacts with CN- to form the complex ion Cu(CN)32-: 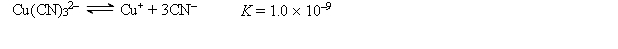 What is the concentration of CN- at equilibrium?
What is the concentration of CN- at equilibrium?
(Multiple Choice)
4.8/5  (43)
(43)
What is the molarity of a sodium hydroxide solution if 25.0 mL of this solution reacts exactly with 22.30 mL of 0.253 M sulfuric acid?
(Multiple Choice)
4.8/5  (32)
(32)
Showing 21 - 40 of 171
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)