Exam 8: Applications of Aqueous Equilibria
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 10-6, Ka2 = 1.0 10-10) is titrated with the following volumes of 1.00 M NaOH.
-400.0 mL of 1.00 M NaOH
(Multiple Choice)
4.8/5  (26)
(26)
A 50.0-mL sample of 2.0 10-4 M CuNO3 is added to 50.0 mL of 4.0 M NaCN. Cu+ reacts with CN- to form the complex ion Cu(CN)32-: 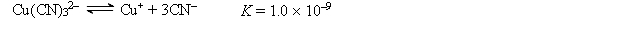 The concentration of Cu+ at equilibrium is
The concentration of Cu+ at equilibrium is
(Multiple Choice)
4.8/5  (36)
(36)
A 10-mL sample of tartaric acid is titrated to a phenolphthalein endpoint with 20. mL of 1.0 M NaOH. Assuming tartaric acid is diprotic, what is the molarity of the acid?
(Multiple Choice)
4.8/5  (32)
(32)
A titration of 100.0 mL of 1.00 M malonic acid (H2A) was done with 1.00 M NaOH. For malonic acid, Ka1 = 1.49 10-2, Ka2 = 2.03 10-6.
-Calculate [H+] after 150.0 mL of 1.00 M NaOH has been added.
(Multiple Choice)
4.9/5  (47)
(47)
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 10-6, Ka2 = 1.0 10-10) is titrated with the following volumes of 1.00 M NaOH.
-300.0 mL of 1.00 M NaOH
(Multiple Choice)
4.9/5  (38)
(38)
If 22 mL of 0.50 M HCl is added to 112 mL of 0.20 M NaOH, what is the final pH?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following compounds has the lowest solubility, in moles per liter, in water at 25°C?
(Multiple Choice)
4.8/5  (44)
(44)
A solution of hydrochloric acid of unknown concentration was titrated with 0.11 M NaOH. If a 150-mL sample of the HCl solution required exactly 12 mL of the NaOH solution to reach the equivalence point, what was the pH of the HCl solution?
(Multiple Choice)
4.8/5  (34)
(34)
It is observed that 7.5 mmol of BaF2 will dissolve in 1.0 L of water. Use these data to calculate the value of Ksp for barium fluoride.
(Multiple Choice)
4.8/5  (34)
(34)
The concentration of Al3+ in a saturated solution of Al(OH)3 at 25°C is 5.2 10-9 M. Calculate the Ksp for Al(OH)3.
(Multiple Choice)
4.9/5  (43)
(43)
Consider the following information about the diprotic acid ascorbic acid (H2As for short, molar mass = 176.1). 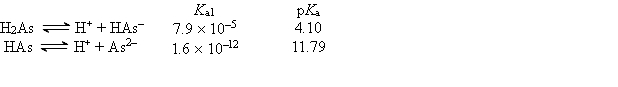 The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 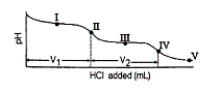 -What major species is(are) present at point III?
-What major species is(are) present at point III?
(Multiple Choice)
4.8/5  (41)
(41)
Showing 161 - 171 of 171
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)