Exam 8: Applications of Aqueous Equilibria
Exam 2: Atoms, Molecules, and Ions61 Questions
Exam 3: Stoichiometry100 Questions
Exam 4: Chemical Reactions and Solutions Stoichiometry93 Questions
Exam 5: Gases113 Questions
Exam 6: Chemical Equilibrium71 Questions
Exam 7: Acids and Bases119 Questions
Exam 8: Applications of Aqueous Equilibria171 Questions
Exam 9: Energy, Enthalpy, and Thermochemistry81 Questions
Exam 10: Spontaneity, Entropy, and Free Energy138 Questions
Exam 11: Electrochemistry85 Questions
Exam 12: Quantum Mechanics and Atomic Theory120 Questions
Exam 13: Bonding: General Concepts135 Questions
Exam 14: Covalent Bonding: Orbitals76 Questions
Exam 15: Chemical Kinetics119 Questions
Exam 16: Liquids and Solids106 Questions
Exam 17: Properties of Solutions99 Questions
Exam 18: The Representative Elements122 Questions
Exam 19: Transition Metals and Coordination Chemistry91 Questions
Exam 20: The Nucleus: a Chemists View68 Questions
Exam 21: Organic and Biochemical Molecules118 Questions
Select questions type
Which is the correct mathematical expression for the molar solubility (x) in moles per liter of Fe3(PO4)2?
(Multiple Choice)
4.7/5  (26)
(26)
A certain indicator HIn has a pKa of 9.00, and a color change becomes visible when 7.00% of it is In-. At what pH is this color change visible?
(Multiple Choice)
4.8/5  (39)
(39)
Calculate the pH when 200.0 mL of a 1.00 M solution of H2A (Ka1 = 1.0 10-6, Ka2 = 1.0 10-10) is titrated with the following volumes of 1.00 M NaOH. 100.0 mL of 1.00 M NaOH
(Multiple Choice)
4.9/5  (34)
(34)
Silver chromate, Ag2CrO4, has a Ksp of 9.0 10-12. Calculate the solubility, in moles per liter, of silver chromate.
(Multiple Choice)
4.9/5  (40)
(40)
A solution is prepared by mixing hydrazoic acid (HN3) and NaN3 and then allowed to come to equilibrium. Upon addition of sulfuric acid
(Multiple Choice)
4.9/5  (33)
(33)
What volume of 0.0100 M NaOH must be added to 1.00 L of 0.0500 M HOCl to achieve a pH of 8.00? Ka for HOCl is 3.5 10-8.
(Multiple Choice)
4.8/5  (25)
(25)
Consider the following information about the diprotic acid ascorbic acid (H2As for short, molar mass = 176.1). 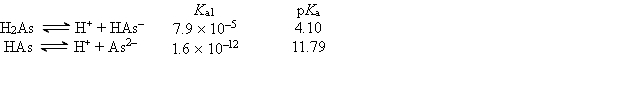 The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 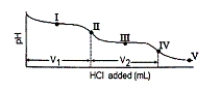 -What is the pH at point I (V1/2 HCl added)?
-What is the pH at point I (V1/2 HCl added)?
(Multiple Choice)
4.7/5  (33)
(33)
What combination of substances will give a buffered solution that has a pH of 5.05? Assume each pair of substances is dissolved in 5.0 L of water. (Kb for NH3 = 1.8 10-5; Kb for C5H5N = 1.7 10-9)
(Multiple Choice)
4.9/5  (35)
(35)
The solubility, in moles per liter, of Ag2CrO4 is 1.3 10-4 M at 25°C. Calculate Ksp for this compound.
(Multiple Choice)
4.7/5  (39)
(39)
Calculate the pH at the equivalence point for the titration of 1.0 M ethylamine, C2H5NH2, by 1.0 M perchloric acid, HClO4. (pKb for C2H5NH2 = 3.25)
(Multiple Choice)
4.8/5  (46)
(46)
In a solution prepared by adding excess PbI2(s) [Ksp = 1.4 10-8] to water, [I-] at equilibrium is
(Multiple Choice)
4.8/5  (37)
(37)
For ammonia, Kb is 1.8 10-5 . To make a buffered solution with pH 10.0, the ratio of NH4Cl to NH3 must be
(Multiple Choice)
4.8/5  (29)
(29)
How many moles of Ca(NO3)2 must be added to 1.0 L of a 0.12 M HF solution to begin precipitation of CaF2(s)? For CaF2, Ksp = 4.0 10-11.
(Multiple Choice)
4.9/5  (41)
(41)
A 200.0-mL sample of the weak acid H3A (0.100 M) is titrated with 0.200 M NaOH. What are the major species at each of the following points in the titration? (Water is always assumed to be a major species.)
-After 350.0 mL of 0.200 M NaOH is added
(Multiple Choice)
4.8/5  (41)
(41)
A 65.5-mL sample of 0.14 M HNO2 (Ka = 4.0 10-4) is titrated with 0.11 M NaOH. What is the pH after 26.8 mL of NaOH has been added?
(Multiple Choice)
4.9/5  (36)
(36)
Calculate the solubility of Ag2SO4 [Ksp = 1.2 10-5] in a 2.0 M AgNO3 solution.
(Multiple Choice)
4.8/5  (39)
(39)
Calculate the solubility of Ca3(PO4)2(s) (Ksp = 1.3 10-32) in a 1.0 10-2 M Ca(NO3)2 solution.
(Multiple Choice)
4.8/5  (29)
(29)
The concentration of Mg2+ in seawater is 0.052 M. At what pH will 99% of the Mg2+ be precipitated as the hydroxide? (Ksp for Mg(OH)2 = 8.9 10-12)
(Multiple Choice)
4.8/5  (37)
(37)
You have two salts, AgX and AgY, with very similar Ksp values. You know that Ka for HX is much greater than Ka for HY. Which salt is more soluble in acidic solution?
(Multiple Choice)
4.8/5  (38)
(38)
A titration of 100.0 mL of 1.00 M malonic acid (H2A) was done with 1.00 M NaOH. For malonic acid, Ka1 = 1.49 10-2, Ka2 = 2.03 10-6.
-Calculate [H+] after 300.0 mL of 1.00 M NaOH has been added.
(Multiple Choice)
4.7/5  (36)
(36)
Showing 121 - 140 of 171
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)