Exam 4: Introduction to Gases
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations49 Questions
Exam 4: Introduction to Gases49 Questions
Exam 5: Atomic Theory: the Nuclear Model of the Atom46 Questions
Exam 6: Chemical Nomenclature43 Questions
Exam 7: Chemical Formula Relationships44 Questions
Exam 8: Chemical Reactions43 Questions
Exam 9: Chemical Change51 Questions
Exam 10: Quantity Relationships in Chemical Reactions40 Questions
Exam 11: Atomic Theory: the Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications45 Questions
Exam 15: Gases, liquids, and Solids45 Questions
Exam 16: Solutions43 Questions
Exam 17: Acid-Base Proton-Transferreactions45 Questions
Exam 18: Chemical Equilibrium44 Questions
Exam 19: Oxidation-Reduction Redoxreactions44 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
On a day when atmospheric pressure is 14.6 psi,a tire pressure gauge is used to measure the pressure of an automobile tire and it registers 42.0 psi.What is the actual air pressure inside the tire?
(Multiple Choice)
4.9/5  (34)
(34)
The pressure on 474 mL of a gas is changed from 726 mm Hg to 622 mm Hg.What is the volume at the new pressure?
(Multiple Choice)
4.9/5  (44)
(44)
The pressure of a gas is 817 torr at 55.0°C.The quantity of the gas is fixed and the volume remains constant as the pressure is adjusted to 6.00 * 102 torr.What is the resulting temperature of the gas?
(Multiple Choice)
4.8/5  (35)
(35)
A container holds 34.3 m3 of gas at 65.0°C.If pressure remains constant,what will the volume be if the temperature falls to 25 °C?
(Multiple Choice)
4.9/5  (31)
(31)
A gas occupies 4.71 liters at 62 °C and 795 torr.What volume will it occupy if it is cooled to 17 °C and compressed to 911 torr?
(Multiple Choice)
4.8/5  (45)
(45)
A gas sample originally occupies 436 mL at 24 °C.When the volume is expanded to 612 mL and the temperature is increased to 97 °C,the pressure becomes 526 mm Hg.What was the original pressure?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following correctly describes the relationship between the volume and temperature of a fixed quantity of an ideal gas at constant pressure?
(i)V T
(ii)V 1/T
(iii)V = kT
(iv)V = k(1/T)
(Multiple Choice)
4.9/5  (38)
(38)
The STP volume of an ideal gas is 2.06 m3.Calculate the volume of the sample at 1.75 atm and 27 °C.
(Multiple Choice)
4.9/5  (32)
(32)
Calculate the original volume of a sealed balloon if it was originally at 25 °C and 1.02 atm,and then it was changed to 5.79 L,77 °C,and 1.72 atm.
(Multiple Choice)
4.9/5  (29)
(29)
The volume of a sealed gas balloon is changed from 953 mL to 808 mL at constant temperature.The original pressure was 155 kPa.What is the pressure after the volume is reduced
?
(Multiple Choice)
4.9/5  (34)
(34)
A perfume bottle is opened in a dressing room,and a short while later you detect the odor in the living room.Which of the following features of the ideal gas model best explains this phenomenon?
(Multiple Choice)
4.8/5  (35)
(35)
Consider the following graph. 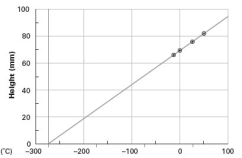 This graph is an illustration of which of the following laws?
This graph is an illustration of which of the following laws?
(Multiple Choice)
4.9/5  (40)
(40)
A cylindrical gas chamber has a piston at one end that can be used to compress or expand the gas.If the gas is initially at 992 torr when the volume is 2.77 L,what will be the volume if the pressure is reduced to 501 torr at constant temperature?
(Multiple Choice)
4.7/5  (48)
(48)
A gas occupies a volume of 3.5 L at 24 °C and 883 mm Hg.What volume will it occupy at STP?
(Multiple Choice)
4.8/5  (44)
(44)
A gas sample occupies 8.15 volume units at 677 pressure units.If possible,find the volume of the gas if the pressure is changed to 278 pressure units.The pressure units are the same in both cases and the temperature is constant.
(Multiple Choice)
4.9/5  (38)
(38)
Consider the following weather map showing the southwestern part of the United States. 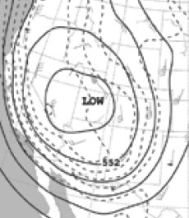 This map seems to indicate that this geographical region:
This map seems to indicate that this geographical region:
(Multiple Choice)
4.8/5  (44)
(44)
A gas with an initial volume of 34.4 mL,measured at -22°C,is heated to 0°C at constant pressure.What is the new volume of the gas?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 21 - 40 of 49
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)