Exam 4: Introduction to Gases
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations49 Questions
Exam 4: Introduction to Gases49 Questions
Exam 5: Atomic Theory: the Nuclear Model of the Atom46 Questions
Exam 6: Chemical Nomenclature43 Questions
Exam 7: Chemical Formula Relationships44 Questions
Exam 8: Chemical Reactions43 Questions
Exam 9: Chemical Change51 Questions
Exam 10: Quantity Relationships in Chemical Reactions40 Questions
Exam 11: Atomic Theory: the Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications45 Questions
Exam 15: Gases, liquids, and Solids45 Questions
Exam 16: Solutions43 Questions
Exam 17: Acid-Base Proton-Transferreactions45 Questions
Exam 18: Chemical Equilibrium44 Questions
Exam 19: Oxidation-Reduction Redoxreactions44 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
Which of the following is the best approximation of the relative densities of air and water,when both are at room temperature?
(Multiple Choice)
4.9/5  (38)
(38)
The temperature of a gas is 23°C.What is its temperature in kelvins?
(Multiple Choice)
4.8/5  (34)
(34)
A gas sample was originally in a 2.50 *102 mL container at 25 °C.The gas was transferred to an evacuated 5.00 * 102 mL container,and the pressure was the same in both containers.What is the temperature of the gas in the larger container?
(Multiple Choice)
4.8/5  (29)
(29)
A scientist builds a "pressure thermometer" by sealing air in a tank.If the pressure inside the tank is 946 mm Hg when it is placed in a boiling water bath at 99 °C,and 749 mm Hg when left on the laboratory bench,what is the temperature in the lab?
(Multiple Choice)
4.8/5  (33)
(33)
A bubble of steam rises to the top of a container of boiling water.Which of the following features of the ideal gas model best explains this phenomenon?
(Multiple Choice)
4.9/5  (35)
(35)
A gas is collected in a container of fixed volume at 25 °C.If the gas exerts a pressure of 685 mm Hg at this temperature,what will the temperature of the gas be when the pressure is 7.60 * 102 mm Hg?
(Multiple Choice)
4.7/5  (35)
(35)
Consider the mercury barometer shown below. 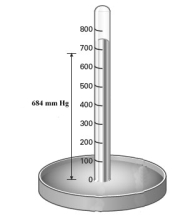 What is the equivalent of pressure reading in torr?
What is the equivalent of pressure reading in torr?
(Multiple Choice)
4.9/5  (39)
(39)
Showing 41 - 49 of 49
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)