Exam 11: Atomic Theory: the Quantum Model of the Atom
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations49 Questions
Exam 4: Introduction to Gases49 Questions
Exam 5: Atomic Theory: the Nuclear Model of the Atom46 Questions
Exam 6: Chemical Nomenclature43 Questions
Exam 7: Chemical Formula Relationships44 Questions
Exam 8: Chemical Reactions43 Questions
Exam 9: Chemical Change51 Questions
Exam 10: Quantity Relationships in Chemical Reactions40 Questions
Exam 11: Atomic Theory: the Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications45 Questions
Exam 15: Gases, liquids, and Solids45 Questions
Exam 16: Solutions43 Questions
Exam 17: Acid-Base Proton-Transferreactions45 Questions
Exam 18: Chemical Equilibrium44 Questions
Exam 19: Oxidation-Reduction Redoxreactions44 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
Which of the following statements is/are incorrect?
(i)The Bohr model successfully predicts energies in the ultraviolet portion of the hydrogen spectrum
(ii)The Bohr model successfully predicts energies in the infrared portions of the sodium spectrum
(iii)The Bohr model associates electron energy with the radius at which an electron orbits the nucleus
(iv)The Bohr model does not explain the failure of an electron to lose energy as it travels a circular path around a nucleus
(Multiple Choice)
4.8/5  (47)
(47)
List the atomic numbers of all atoms that are smaller than atoms of fluorine.
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following elements has the Lewis symbol below,where Sy represents the elemental symbol? 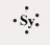
(Multiple Choice)
4.9/5  (36)
(36)
The maximum number of electrons that can be held in the 4f sublevel is...
(Multiple Choice)
4.9/5  (38)
(38)
Consider the following periodic table. 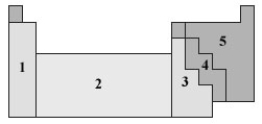 In what numbered section would the elements used in semiconductors be found?
In what numbered section would the elements used in semiconductors be found?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following elements has the Lewis symbol below,where Sy represents the elemental symbol? 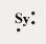
(Multiple Choice)
4.9/5  (38)
(38)
In a given energy level,which of the following types of orbitals will have the lowest energy?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following pairs of atomic numbers belong to elements whose atoms have the highest occupied energy level electron configuration of the form ns2np4?
(Multiple Choice)
4.7/5  (32)
(32)
Which of the following statements is/are correct?
(i)Principal energy levels are identified by the letters s,p,d,and f
(ii)Principal energy levels appear in both the quantum mechanical model of the atom and the Bohr model of the atom
(iii)In general,n = 1 is at lower energy than n = 2,and n = 2 is lower than n = 3,and so on
(iv)The principal energy level is related to electron spin
(Multiple Choice)
4.8/5  (36)
(36)
Showing 41 - 50 of 50
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)