Exam 7: The Quantum-Mechanical Model of the Atom
Exam 1: Units of Measurement for Physical and Chemical Change192 Questions
Exam 2: Atoms and Elements174 Questions
Exam 3: Molecules, Compounds, and Nomenclature187 Questions
Exam 4: Chemical Reactions and Stoichiometry261 Questions
Exam 5: Gases163 Questions
Exam 6: Thermochemistry161 Questions
Exam 7: The Quantum-Mechanical Model of the Atom170 Questions
Exam 8: Periodic Properties of the Elements144 Questions
Exam 9: Chemical Bonding I: Lewis Theory155 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory180 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces144 Questions
Exam 12: Solutions167 Questions
Exam 13: Chemical Kinetics170 Questions
Exam 14: Chemical Equilibrium150 Questions
Exam 15: Acids and Bases156 Questions
Exam 16: Aqueous Ionic Equilibrium173 Questions
Exam 17: Gibbs Energy and Thermodynamics134 Questions
Exam 18: Electrochemistry122 Questions
Exam 19: Radioactivity and Nuclear Chemistry116 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry Ii: Reactions102 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallurgy49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
No two electrons can have the same four quantum numbers. What is this known as?
(Multiple Choice)
4.8/5  (33)
(33)
Calculate the wavelength (in nm) of the red light emitted by a neon sign with a frequency of 4.74 × 1014 Hz.
(Multiple Choice)
4.7/5  (32)
(32)
Calculate the wavelength of light associated with the transition from n = 1 to n = 3 in the hydrogen atom.
(Multiple Choice)
4.9/5  (37)
(37)
An electron ends in orbital n = 4 after a hydrogen atom absorbs a photon with a wavelength of 486 nm. Calculate the initial orbital, ni.
(Multiple Choice)
4.9/5  (34)
(34)
What is the maximum number of p orbitals that are possible in the same level?
(Multiple Choice)
4.7/5  (32)
(32)
Determine the energy change associated with the transition from n = 2 to n = 5 in the hydrogen atom.
(Multiple Choice)
4.9/5  (35)
(35)
Calculate the orbital for the hydrogen atom that contains an electron with an energy of -4.45 × 10-20 J.
(Multiple Choice)
4.9/5  (39)
(39)
Electromagnetic radiation with a wavelength of 525 nm appears as green light to the human eye. The frequency of this light is ________ 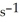 .
.
(Multiple Choice)
4.7/5  (26)
(26)
For hydrogen, what is the wavelength of the photon emitted when an electron drops from a 4d orbital to a 2p orbital in a hydrogen atom? The Rydberg constant is 1.097 × 10-2 nm-1.
(Multiple Choice)
4.8/5  (37)
(37)
Determine the mass of a ball with a velocity of 35.1 m s-1 and a wavelength of 8.92 × 10-34 m.
(Multiple Choice)
4.7/5  (41)
(41)
Place the following types of electromagnetic radiation in order of increasing wavelength. ultraviolet light gamma rays radio waves
(Multiple Choice)
4.8/5  (34)
(34)
On the electromagnetic spectrum, visible light is immediately between two other wavelengths. Name them.
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following colours of electromagnetic radiation has the shortest wavelength?
(Multiple Choice)
4.7/5  (34)
(34)
Calculate the wavelength (in nm) of the blue light emitted by a mercury lamp with a frequency of 6.88 × 1014 Hz.
(Multiple Choice)
4.7/5  (34)
(34)
Determine the energy change associated with the transition from n = 3 to n = 2 in the hydrogen atom.
(Multiple Choice)
4.7/5  (40)
(40)
Calculate the wavelength of an electron (m = 9.11 × 10-28 g) moving at 3.66 × 106 m s-1.
(Multiple Choice)
4.9/5  (35)
(35)
Showing 41 - 60 of 170
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)