Exam 4: Aqueous Reactions and Solution Stoichiometry
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
Which of the following reactions will not occur as written?
(Multiple Choice)
4.8/5  (36)
(36)
A 36.3 mL aliquot of 0.0529 M H2SO4 (aq) is to be titrated with 0.0411 M NaOH (aq). What volume (mL) of base will it take to reach the equivalence point?
(Multiple Choice)
4.9/5  (30)
(30)
Based on the equations below, which metal is the most active?
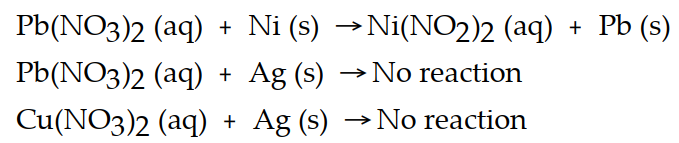
(Multiple Choice)
4.9/5  (31)
(31)
The molarity of an aqueous solution containing 75.3 g of glucose (C6H12O6) in 35.5 mL of solution is .
(Multiple Choice)
4.8/5  (39)
(39)
The concentration (M) of an aqueous methanol produced when 0.200 L of a 2.00 M solution was diluted to 0.800 L is .
(Multiple Choice)
4.9/5  (45)
(45)
What volume (mL) of 7.48 × 10- 2 M perchloric acid can be neutralized with 115 mL of 0.244 M sodium hydroxide?
(Multiple Choice)
4.9/5  (28)
(28)
When gold dissolves in aqua regia, into what form is the gold converted?
(Essay)
4.9/5  (35)
(35)
Silver ions can be precipitated from aqueous solutions by the addition of aqueous chloride:
 Silver chloride is virtually insoluble in water so that the reaction appears to go to completion. How Many grams of solid NaCl must be added to 25.0 mL of 0.366 M AgNO3 solution to completely precipitate the silver?
Silver chloride is virtually insoluble in water so that the reaction appears to go to completion. How Many grams of solid NaCl must be added to 25.0 mL of 0.366 M AgNO3 solution to completely precipitate the silver?
(Multiple Choice)
4.9/5  (40)
(40)
A 31.5 mL aliquot of HNO3 (aq) of unknown concentration was titrated with 0.0134 M NaOH (aq). It took 23.9 mL of the base to reach the endpoint of the titration. The concentration (M) of the acid was .
(Multiple Choice)
4.7/5  (38)
(38)
What volume (L) of 0.250 M HNO3 is required to neutralize a solution prepared by dissolving 17.5 g of NaOH in 350 mL of water?
(Multiple Choice)
4.8/5  (27)
(27)
Consider the following reactions:
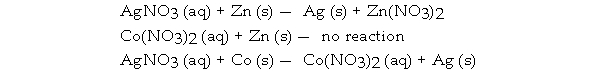 Which is the correct order of increasing activity for these metals?
Which is the correct order of increasing activity for these metals?
(Multiple Choice)
4.9/5  (35)
(35)
Calculate the concentration (M) of sodium ions in a solution made by diluting 40.0 mL of a
0.474 M solution of sodium sulfide to a total volume of 300 mL.
(Short Answer)
4.9/5  (38)
(38)
A strong electrolyte is one that _ completely in solution.
(Multiple Choice)
4.8/5  (34)
(34)
The concentration of species in 500 mL of a 2.104 M solution of sodium sulfate is M sodium ion and M sulfate ion.
(Multiple Choice)
4.9/5  (32)
(32)
How many grams of sodium chloride are there in 55.0 mL of a 1.90 M aqueous solution of sodium chloride?
(Multiple Choice)
4.8/5  (33)
(33)
The net ionic equation for the reaction between aqueous sulfuric acid and aqueous sodium hydroxide is .
(Multiple Choice)
5.0/5  (31)
(31)
How many milliliters of 0.132 M HClO4 solution are needed to neutralize 50.00 mL of 0.0789 M NaOH?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 101 - 120 of 147
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)