Exam 21: Nuclear Chemistry
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
What is the typical percent of uranium- 235 in the enriched UO2 pellets used in nuclear reactors
__________ ?
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
C
In the nuclear transmutation represented by 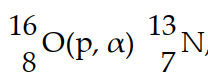 , the emitted particle is .
, the emitted particle is .
Free
(Multiple Choice)
4.8/5  (44)
(44)
Correct Answer:
D
Which of the following correctly represents the transmutation in which a curium- 242 nucleus is bombarded with an alpha particle to produce a californium- 245 nucleus?
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
B
At approximately what number of protons, or neutrons, does the 1:
1 ratio of protons to neutrons start to produce unstable nuclei?
(Multiple Choice)
4.7/5  (34)
(34)
The largest number of stable nuclei have an _ number of protons and an number of neutrons.
(Multiple Choice)
4.9/5  (37)
(37)
The nuclear disintegration series of is the source of radon- 222 in soil.
(Multiple Choice)
4.9/5  (35)
(35)
A rock contains 0.313 mg of lead- 206 for each milligram of uranium- 238. The half- life of for the decay of uranium- 238 to lead- 206 is 4.5 × 109 yr. The rock was formed yr ago.
(Multiple Choice)
4.9/5  (40)
(40)
Potassium- 40 decays to argon- 40 with a half- life of 1.27 × 109 yr. The age of a mineral sample that has a mass ratio of 40Ar to 40K of 0.812 is _ _ yr.
(Multiple Choice)
4.9/5  (36)
(36)
How many neutrons are emitted when a californium- 249 nucleus (Z=98) is bombarded with a nitrogen- 15 nucleus to produce a 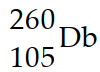 nucleus ?
nucleus ?
(Multiple Choice)
4.8/5  (29)
(29)
What happens to the atomic mass number and the atomic number of a radioisotope when it undergoes alpha emission?
(Essay)
4.9/5  (33)
(33)
If we start with 1.000 g of strontium- 90, 0.908 g will remain after 4.00 yr. This means that the half- life of strontium- 90 is _ yr.
(Multiple Choice)
4.8/5  (26)
(26)
Bombardment of uranium- 238 with a deuteron (hydrogen- 2) generates neptunium- 237 and
Neutrons.
(Multiple Choice)
4.9/5  (39)
(39)
Carbon- 11 is used in medical imaging. The half- life of this radioisotope is 20.4 min. What percentage of a sample remains after 60.0 min?
(Multiple Choice)
4.8/5  (31)
(31)
Which one of the following is a correct representation of a beta particle?
(Multiple Choice)
4.8/5  (38)
(38)
Who is credited with first achieving fission of uranium- 235 ?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 1 - 20 of 128
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)