Exam 8: Mixtures, Solution Concentrations, and Diffusion
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
The chart below illustrates the composition of air.According to this chart 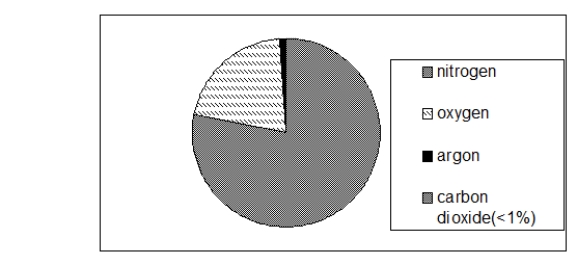
(Multiple Choice)
4.7/5  (42)
(42)
There are many mixtures in the body.The most common ______ for these mixtures is water.
(Multiple Choice)
4.8/5  (39)
(39)
Which statement explains why whole blood has characteristics of a solution, a colloid, and a suspension?
(Multiple Choice)
4.9/5  (31)
(31)
The function of dialysate is similar in function to which part of the diagram below? 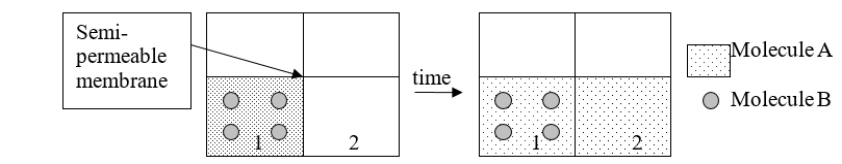
(Multiple Choice)
4.7/5  (30)
(30)
A solution is prepared by dissolving 1.5 g of NaCl in enough water to produce a total solution volume of 750 mL.What is the concentration of the solution in % m/v?
(Multiple Choice)
4.9/5  (30)
(30)
A technician needs to make 500 mL of a 50 mM solution.How many milliliters of the 200 mM stock solution are needed?
(Multiple Choice)
4.7/5  (41)
(41)
What volume of a 6.0% solution of ethanol contains 3.0 g of ethanol?
(Multiple Choice)
4.8/5  (34)
(34)
How many grams of NaCl are in 350 mL of normal saline (0.9% NaCl)?
(Multiple Choice)
4.8/5  (27)
(27)
A technician adds 0.250 L of 200 mM buffer stock solution to a volumetric flask and dilutes the stock to 1.0 L.What is the final concentration of the solution?
(Multiple Choice)
5.0/5  (34)
(34)
In hemodialysis, the solution used in the dialyzer, the dialysate is __________ with respect to blood.
(Multiple Choice)
4.9/5  (30)
(30)
Jane Doe's blood test showed that her iron is 125 mg/dL.How many grams per liter, g/L, is 125 mg/dL?
(Multiple Choice)
4.9/5  (34)
(34)
It is normal to have about 100 mM (mmol/L)of chloride (Cl-)in the blood.How many meq is this?
(Multiple Choice)
4.8/5  (36)
(36)
Cephadrine is one of many antibiotics that act by inhibiting bacterial cell wall synthesis.If a typical daily dosage is 560 mg, how many milliliters of 80.mg/mL suspension of cephadrine should be administered daily?
(Multiple Choice)
4.8/5  (39)
(39)
When sodium chloride dissolves in water, it forms _______ with water molecules.
(Multiple Choice)
4.8/5  (25)
(25)
The concentration of an adrenalin chloride solution, used to treat acute allergic responses, is 15 mg in 30.mL.Express this concentration in units of mg/mL (milligrams per milliliter).
(Multiple Choice)
4.7/5  (40)
(40)
How many grams of dextrose are in 100 mL of D5W (5.0% dextrose)?
(Multiple Choice)
4.8/5  (27)
(27)
When MgCl2 dissolves in water, it produces Mg2+ and Cl- ions.Which statement BEST describes the interaction between Cl- and water?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 21 - 40 of 97
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)