Exam 8: Mixtures, Solution Concentrations, and Diffusion
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
How many moles of magnesium chloride (MgCl2)are in 1.31 L of 0.38 M MgCl2?
(Multiple Choice)
4.9/5  (41)
(41)
A typical ______ contains aggregates of atoms, molecules, or ions.It can also contain large molecules distributed throughout the medium.
(Multiple Choice)
4.9/5  (30)
(30)
How many ions of each type are produced when Na3PO4 is dissolved in aqueous solution?
(Multiple Choice)
4.8/5  (47)
(47)
A _______ settles after mixing and contains visible particles.
(Multiple Choice)
4.9/5  (39)
(39)
In a colloidal dispersion, the major component is called the
(Multiple Choice)
4.8/5  (36)
(36)
A wine label states that it is 12 proof, meaning that it contains 6% ethanol.According to this information
(Multiple Choice)
4.7/5  (41)
(41)
A solute dissolving in a solvent is a _______ change because _______broken in the process.
(Multiple Choice)
4.9/5  (35)
(35)
A student adds 0.250 L of 200 mM buffer stock solution to a volumetric flask and dilutes the stock to 2.0 L.What is the final concentration of the solution?
(Multiple Choice)
4.9/5  (29)
(29)
How many moles of glucose are in 0.82 L of a 0.13 M glucose solution?
(Multiple Choice)
4.9/5  (34)
(34)
According to a blood test, a patient has a blood calcium concentration of 9.7 mg/dL.Express this concentration in units of mg/mL (milligrams per milliliter).
(Multiple Choice)
4.8/5  (29)
(29)
Capsaicin is responsible for the heat of peppers.It is common practice to fry capsaicin in oil prior to eating.Which part of the capsaicin molecule forms an intermolecular attractive force with oil, a nonpolar solvent? 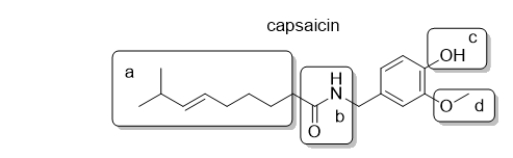
(Multiple Choice)
4.8/5  (29)
(29)
Does ethanol, a polar organic molecule that can hydrogen bond, dissolve in water?
(Multiple Choice)
5.0/5  (29)
(29)
Which statement describes one way in which osmosis and dialysis are similar?
(Multiple Choice)
4.8/5  (34)
(34)
A 1% epinephrine solution is delivered by inhalation to treat asthma.This concentration is equivalent to
(Multiple Choice)
4.7/5  (39)
(39)
Jane Doe has a blood glucose level of 92 mg/dL.How many grams of glucose does she have in 1 L of her blood?
(Multiple Choice)
4.9/5  (38)
(38)
Showing 81 - 97 of 97
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)