Exam 8: Mixtures, Solution Concentrations, and Diffusion
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
Consider solutions A and B separated by a semipermeable membrane.To which volume would pressure need to be applied to prevent osmosis from occurring? 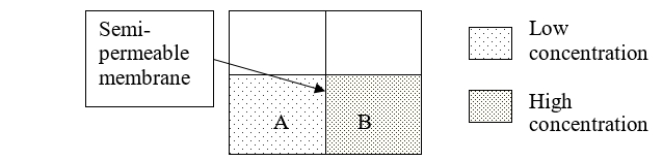
(Multiple Choice)
4.9/5  (38)
(38)
Cephadrine is one of many antibiotics that act by inhibiting bacterial cell wall synthesis.If a typical daily dosage is 660 mg, how many milliliters of 75 mg/mL suspension of cephadrine should be administered daily?
(Multiple Choice)
4.9/5  (35)
(35)
How many grams of glucose should be weighed out to make 1 liter of a rehydration solution that contains 75 mmol of glucose (the molar mass of glucose is 180 g)?
(Multiple Choice)
4.8/5  (29)
(29)
A _______ has components that are evenly distributed throughout the mixture.
(Multiple Choice)
4.9/5  (38)
(38)
Which picture BEST represents ethanol dissolved in water? Note that dashed lines represent hydrogen bonds. 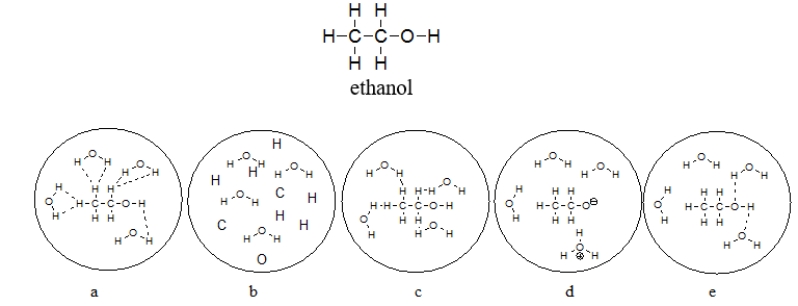
(Multiple Choice)
4.7/5  (33)
(33)
Which figure BEST represents the dissolution of MgCl2 in water? 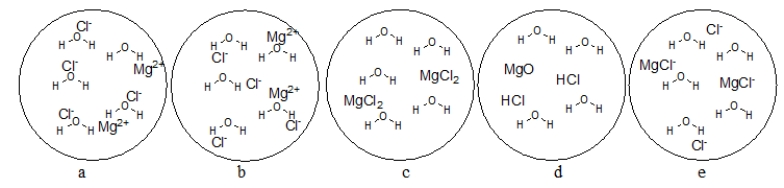
(Multiple Choice)
4.9/5  (35)
(35)
Drinking water from a well is discovered to have an arsenic concentration of 3.4 × 10-4 g of arsenic per liter.Does this value exceed the permissible level of arsenic in drinking water of 10 ppb?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following does NOT happen when the kidneys stop functioning?
(Multiple Choice)
4.9/5  (35)
(35)
A chemist needs 500 mL of a 20 mM phosphate buffer.The stock solution of the buffer is 100 mM.How much stock solution should be used to make the 20 mM phosphate buffer?
(Multiple Choice)
4.8/5  (38)
(38)
A nurse needs 2.0 L of D5W, 5.0 % dextrose.The stock solution of dextrose is 20%.How much stock solution should be used to make the D5W?
(Multiple Choice)
4.8/5  (31)
(31)
Red blood cells are placed into three solutions, each solution a different concentration of NaCl.One solution is isotonic, one is hypotonic, and one is hypertonic with red blood cells.Solution A is _______, and solution C is _______ with the red blood cells. 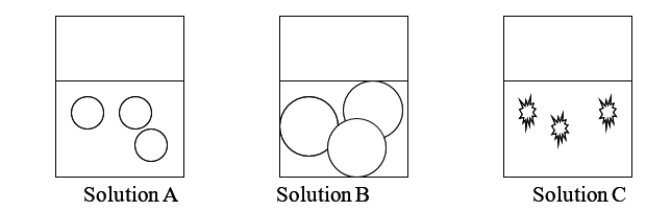
(Multiple Choice)
4.8/5  (31)
(31)
To make 1.0 L of a 10.g NaCl/L solution, 10.g of NaCl is weighed and poured into a volumetric flask.What is the next step?
(Multiple Choice)
4.9/5  (33)
(33)
According to a blood test, a patient has a blood iron value of 125 μg/dL.This value means that
(Multiple Choice)
4.8/5  (30)
(30)
A vial contains 5 mL of 1% aqueous lidocaine, a dental anesthetic.In this solution
(Multiple Choice)
4.9/5  (33)
(33)
A biologist needs 500 mL of a 10 mM phosphate buffer.The stock solution of the buffer is 100 mM.How much stock solution should be used to make the 10 mM phosphate buffer?
(Multiple Choice)
4.7/5  (35)
(35)
Showing 61 - 80 of 97
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)